|
In this final part of my series on the
food of coral reefs and of corals, I literally tackle an enormous
subject. In spite of that, it will become apparent that it
describes a relatively unimportant source of nutrition to
corals (for a number of reasons). The production, decompositions,
flux and uptake of dissolved nutrients involves not just corals,
but the whole coral reef, and involves everything from sediments
to the water column, and from bacteria to fishes. Therefore,
the introduction to this article is at best limited to a most
brief overview of the subject.
Introduction
Dissolved nutrients are composed of both
organic and inorganic forms. By "nutrients" I mean
that any molecules that can be taken up and used as "food"
- catabolized or used as building locks for metabolic processes.
Dissolved organic material, commonly known as DOM, must by
definition, contain carbon, and often contains hydrogen, oxygen
and nitrogen, as well. Dissolved inorganic material is composed
of those substances lacking the carbon component, and may
be simple free ions, such as nitrate (NO3-),
as well as other types of material.
One of the difficulties in ascertaining
the amount of dissolved organic materials in seawater, or
their uptake by various organisms, is that the dissolved organics
tend to form films at water/air interfaces and also form colloidal
aggregates (particles between 1nm and 1µm in diameter)
in the water column, often by association with iron or other
metal compounds and "turning into" particulate material.
The colloids, finely divided particles (a few millionths of
a millimeter) dispersed within a continuous medium in a manner
that prevents them from being filtered easily or settled rapidly,
can also attract various inorganic molecules, including trace
metals, leading to a somewhat complex situation. In one sense,
it may allow larger colloids to be included in the fraction
of particulate organic matter (while, conversely, some particulate
matter may be colloids of dissolved materials). This also
makes it difficult to assess the true nature of uptake, and
whether or not the uptake is a generalized process or if specific
components are targeted, utilized, required, or even toxic
and incidental components of otherwise desirable materials.
Generally speaking, "dissolved" organic material
is defined as fractions that pass through a 0.45µm filter.
Furthermore, dissolved material may be adsorbed from water
onto carbonates, clays, and particulate material that is not
only present in seawater, but also may be a significant reservoir
of both organic and inorganic constituents. True organic material
in solution and not in the colloidal state is generally less
than 0.1µm in size. As an example of the plethora of
materials in the difficult to assess size class, many bacteria
are between 0.25 and 1µm in size, and the common phytoplankton,
Nannochloropsis oculata is 2-3µm in size. Thus,
bacteria, and a partially consumed or degraded phytoplankton
particles, clearly not dissolved organic material, may be
included in this category simply by virtue of size and methods
that screen DOM by filtration. To further complicate the matter,
bacteria generally mediate the events. For example, dissolved
organic carbon initiates bacterially mediated aggregation
of detrital (particulate) material. The bacteria, using the
material as a food source may form films, or they may cement
the material together (Figure 1).
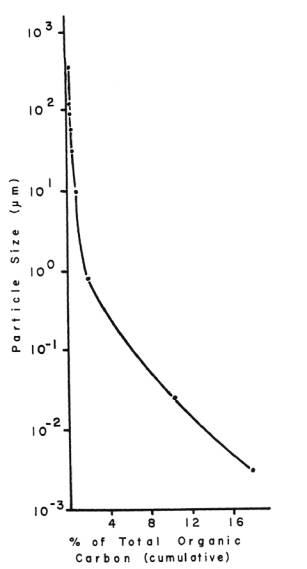 |
Figure 1. The percentages of dissolved carbon belonging
to different size classes. The majority of dissolved organic
carbon (DOC) is truly "dissolved," although significant
proportions exists in larger size classes, such as in colloids.
The pattern seen here for carbon is similar to that seen for
most other elements and compounds, as well.
Microbial use of dissolved organic material
adsorbed onto carbonate sand is responsible for the not infrequent
"cementing" of sand beds in aquaria. Other organic
aggregates may occur with the absorption of materials onto
the surface of air bubbles (the general principle behind protein
skimming, whereby dissolved proteins are removed by adherence
to foam produced by the skimmer). As a side note, it is often
said that the foam produced by wave action acts like "natural
skimming." This is frequently countered by those saying
this analogy has never been proven. Well, it has been proven
- often (see references at the end of this article). A final
important mode or organic aggregation, and perhaps most applicable
to coral reefs, occurs through the production of mucus by
many organisms. Mucus consists of mainly sugars and glycoproteins
- soluble materials in and of themselves. However, the formation
of mucus and its release in a matrix of chains of these materials,
may result in a particulate material. This material is both
utilized directly by many organisms, and also forms the basis
for a predominant fraction of the particulate "marine
snow" on reefs. In the latter situation, it is utilized
and loosely adhered together by microbes and subsequently
ensnares other particulate material, forming even larger accumulations
that are clearly no longer "dissolved' by any means (Figure
2).
Click
for larger image.
The Levels and Production of Dissolved Nutrients
Nutrients are brought to the reef via numerous
pathways. First, they are brought with oceanic water; either
by thermohaline upwellings or currents and waves driven by
wind and tides. Groundwater may be another important nutrient
source. Land based nutrients are also produced and washed
seaward to reefs from rains, and river and coastal discharges.
More recently, enhanced nutrient levels from anthropogenic
land-based (sewage discharge, coastal development, land clearing
and agriculture), and marine-based sources (ships, etc.) have
produced a somewhat disturbing pattern of eutrophication.
Nutrients are also formed allocthonously
(produced by the reef) by the excess secretions and excretions
of the organisms (feces, mucus, etc.). Dissolved nutrients
"leak" from algae, plankton, and other organisms,
as well. All dead and decomposing matter eventually makes
its way back into the nutrient broth of seawater as a product
of microbial degradation, and nitrogen is also "fixed"
by certain classes of bacteria and blue-green algae (cyanobacteria).
These processes occur throughout the reef, from aerobic surfaces
to deep anoxic sediments. It is primarily the sediments that
form the "action areas" of decomposition and remineralization
or organic materials. Moreover, adjacent communities, such
as mangroves, estuaries, and seagrass areas provide large
areas of enriched production and nutrient export to coral
reefs.
Still, the abundance of life on coral reefs,
living in clear tropical waters relatively devoid of dissolved
nutrients, could not be sustained by this resource alone (Table
1).
| Nutrient |
Temperate
|
Tropical
|
|
Nitrate
|
2.0-5.0
|
0.1-0.3
|
|
Ammonia
|
<1.0
|
0.2-0.5
|
|
Phosphate
|
0.5-2.0
|
<0.3
|
|
Silicate
|
<0.2
|
<2.0
|
|
Dissolved
Organic Phosphate
|
<1.3
|
<0.15
|
|
Dissolved
Organic Nitrogen
|
5.0-20.0
|
3.0-13.8
|
|
Dissolved
Organic Carbon
|
1500-2000
|
500-800
|
|
Table
1. Dissolved Nutrient Species in temperate and reef
waters (from Crossland 1983).
|
As such, there is a tight recycling of
nutrients within the reef community that includes the well-known
symbioses between photosynthetic organisms (like zooxanthellae)
and animal hosts (clams, sponges, corals, etc.) The dynamics
of inorganic nutrient cycling on coral reefs have been studied
extensively, and yet are still unknown in many aspects. They
are, however, beyond the scope of this article, and I confine
any further discussion to the set of tables and figures below.
Dissolved organic material on reefs is
much less studied (despite the plethora of references at the
end of this article) and is probably underestimated. The low
levels in oceanic waters are boosted primarily by phytoplankton
excretion and then again from the products of benthic algae
and corals. In fact, reef waters are 30-40% higher in DOM
than oceanic waters, and this makes sense since 5-40% of the
photosynthetic production of phytoplankton, algae, and corals
is lost primarily as glycine, amino acids, sugars, vitamins,
fatty acids, and phenols.
The Uptake of Dissolved Materials
Given the introduction above, it may not
be surprising that deciphering reports and studies on the
uptake of dissolved materials by reef organisms includes assessing
whether the reported "use" also includes other forms,
such as bacteria and particulate materials. Both of these
are well known to be removed and use as sources of heterotrophic
nutrition that should be considered separate from the use
of dissolved materials. In fact, many early studies simply
lumped bacteria into the DOM fraction and ignored particulates
within the "dissolved" size fraction, and instead
dealt with particulates above a certain size class.
In general, the use of dissolved material
requires a means of uptake. For the larger size classes, including
some of the colloids, phagocytosis is probably utilized. In
this method, material is encircled by the cell membrane of
an organism's cells and is "pinched off," and is
then located in a membrane-surrounded compartment called a
vacuole. The vacuole will fuse with other compartments containing
enzymes, and the material is digested into components able
to be used by the cell. Smaller dissolved material probably
enters the cell by pinocytosis that doesn't usually involve
the formation of a vacuole, though the basic uptake processes
are similar.
In terms of determining the degree to which
organisms may use dissolved material as significant source
of nutrition, one may examine the surface area of the outer
cell membranes exposed to such materials. In general, the
larger the absorptive area, the more likely organisms are
to depend on absorption. Some cell surfaces are covered with
many finger-like processes called microvilli that greatly
increase the surface area of the membrane. Furthermore, the
presence of cilia is often a good indicator of absorptive
surfaces. Perhaps not surprisingly, corals have extensive
microvilli and cilia.
Dissolved "Nutrients"
What are nutrients? They are substances
that are required or produced for use in the normal metabolic
functioning of an organism or cell. Depending on the organism,
they may include unusual substances, such as vanadium or yttrium.
However, basic organic constituents are typically far less
exotic. Thus, the term dissolved "nutrients" typically
refers to common metabolic building blocks such as carbon,
nitrogen, and phosphorus. Despite the heavy uptake of elements
such as calcium by calcifying organisms, calcium is not normally
considered a major "nutrient." However, trace amounts
of calcium are used in cell function and metabolism of most
organisms. It may be notable, though, that dissolved organic
material, and inorganic ions such as magnesium, may interfere
significantly with the precipitation of calcium carbonate
(Chave and Suess 1970; Meyers and Quinn 1971).
Because this is a column that focuses on
corals, I will constrain the remainder of this article to
the use of dissolved major nutrients by corals. It is not
my intent to discuss every case where a species might have
unusual requirements (for example, iodine in some gorgonians),
nor will I address the many theoretical considerations that
will likely plague some minds after reading the remainder
of this column. Rather, I confine the topic to information
that is available and reasonably well document with an emphasis
on practical considerations that are part of maintaining corals
in aquaria.
Uptake of Dissolved Material by Corals
Phosphorus
Early studies of phosphorus flux across
coral reefs showed little net uptake or loss by coral and
algae, but mostly a change in the form of phosphorus (Pilson
and Betzer 1973). However, Atkinson (1987) later found that
the reef flat community, mainly algae and corals, were taking
up phosphorus as fast as they were known to be able to do,
and the main limitation to the rate of uptake was the low
availability of phosphorus in the environment (Table 2).
|
Process |
Important organisms
or chemical phenomena |
Comments
|
|
Assimilation
|
Macrophytes
Foraminiferans
Corals
Bacteria
Microalgae
|
Studied,
but not well quantified
|
|
Excretion
and Hydrolysis
|
Grazers
Filter-feeders
Bacteria
Protozoans
|
Studied,
but not well quantified
|
|
Precipitation,
adsorption and chemisorption
|
Physicochemical
sorption
Corprecipitation
with calcium carbonate
|
Studied,
but not well quantified
|
|
Dissolution
and desorption
|
Physicochemical
sorption stimulated by bioturbation
|
Not
well studied or quantified
|
Table
2. Major phosphorus processes and important organims or
chemical phenomena
involved (from D'Elia and Wiebe 1990).
Corals are known to take up phosphorus
in solution. However, this process is thought to be mainly
a function of zooxanthellae uptake rather than the coral polyp,
since only zooxanthellate corals uptake phosphorus, and this
activity only takes place during the day (Yonge and Nichols
1931; d'Elia 1977). It was proposed that phosphatases found
in tridacnid clams were involved in digesting zooxanthellae
by the animal (Fitt and Trench 1983), but more recent work
suggests that it is likely that their presence is a zooxanthellae
response to phosphate limitation by the host polyp with phosphorus
uptake (as well as other nutrients) controlled by the algal
membrane (Jackson et al. 1989, Rand et al. 1993). Still, phosphorus
is present in very low amounts in the reef environment (Figure
3), and recycling of phosphorus between host and algae, even
if ultimately controlled by the alga, is likely a necessary
requirement for the survival of both partners of the symbiosis
in order to acquire adequate phosphorus from the environment
(D'Elia 1977).
Click
for larger image.
Corals have been previously found to "prefer"
particulate sources of phosphorus over dissolved forms (Sorokin
1973). Much of the dissolved fraction of phosphorus on reefs
may be adsorbed onto calcareous substrates such as sand and
the reef material, and can be liberated by dissolution.
Nitrogen
Despite the view above that phosphorus
may indeed be a limiting nutrient source on coral reefs, far
more study and far more attention has been paid to the role
of nitrogen on coral reefs and the role of dissolved forms
of nitrogen to reef coral (Figure 4).
|
|
|
Figure
4. Dissolved inorganic nitrogen levels from various
reefs in micromoles/liter (from D'Elia and Wiebe 1990).
Click for larger image.
|
It has also been widely stated and often
accepted the nitrogen may be the primary limiting nutrient
to reef organisms, although the view that phosphorus truly
limits corals has been well-made (Miller and Yellowlees 1989).
It is also interesting that despite the lack of dissolved
nitrogen on coral reefs, reefs are generally sources of net
export of nitrogenous compounds to oceanic waters!
|
Nutrient
|
windward
|
leeward
|
algae
|
corals
|
net
|
|
Nitrate
|
Export
(day)
No
change (night)
|
Uptake
(day)
Export
(night)
|
Uptake
|
Export
|
No
change
|
| Ammonia |
Uptake
(day)
No
change (night) |
Uptake
(day)
No
change (night) |
Export |
Export
|
Uptake/Export
|
|
Nitrite
|
Export
|
Uptake
|
|
|
Uptake
|
|
Organic N
|
Export
|
Export
|
|
Export
|
Export
|
|
Inorganic P
|
Uptake
|
Export
|
Uptake
|
|
Negligible
|
|
Organic P
|
|
|
|
|
Export
|
|
Silicate
|
Uptake (day)
Export
(night)
|
Uptake (day)
|
|
|
Uptake
|
|
Table
3. Gross Nitrogen and Phosphorus flux across several
reefs
(summarized from Crossland 1983).
|
As with phosphorus, nitrogen appears to
be highly recycled within the reef community; such recycling
occurs both between the organisms in the community and within
each of the corals through recycling between host polyp and
algal symbiont (Rahav et al. 1989). On reefs, the most abundant
form of dissolved nitrogen is ammonium, and not nitrate as
is the case in aquaria. Also similar to the case with phosphorus,
azooxanthellate corals appear to have a net export of nitrogen
whereas zooxanthellate coral appears to have a net uptake
of dissolved nitrogen. Nitrogen fixation and denitrification
also occurs, and this is thought to be result of bacterially
and cyanobacterially mediated events that take place not only
on the surface of corals (Wafar et al. 1990), but throughout
the microbially productive surfaces and sediments on and surrounding
coral reefs (Webb et al. 1975). Because sediments are enriched
with organic material, there is generally a net efflux of
dissolved nitrogen from sediments and other pore waters (coral
skeletons, live rock) into the water column. This may also
lead to locally higher levels in areas with lower water circulation.
Nitrogen fixation is thought to be the most important source
of dissolved nitrogen to coral reefs, while denitrification
is thought to be the major source of nitrogen loss. A tremendous
description of the complete nitrogen cycle on coral reefs
is found in D'Elia and Wiebe (1990). Most aquarists are only
familiar with the limited process of nitrification and denitrification,
but this chapter describes all aspects of the nitrogen cycle
in detail (Table 4).
|
Process |
Important Organisms |
Comments |
|
Nitrogen
fixation
|
Cyanobacteria
|
Well
studied, rates well quantified
|
|
Ammonification
|
Grazers
Detritivores
Filter-feeders
Bacteria
|
Studied,
but not well understood or quantified
|
|
Nitrification:
ammonia
|
Bacteria
|
Studied
and quantified
|
|
Nitrification:
nitrite
|
Bacteria
|
Studied
and quantified
|
|
Dissimilatory
nitrate reduction and denitrification
|
Bacteria
|
Not
well studied or quantified
|
|
Assimilatory
nitrite reduction
|
Macrophytes
Corals
Foraminiferans
Bacteria
|
Well
studied and quantified
|
|
Assimilatory
nitrate reduction
|
Macrophytes
Corals
Foraminiferans
Bacteria
|
Well
studied and quantified
|
|
Immobilization
and assimilation
|
Corals
Foraminiferans
Bacteria
|
Well
studied and quantified
|
Table
4. Major nitrogen cycle processes and important organisms
involved (from D'Elia and Wiebe 1990).
Corals are able to take up various forms
of both organic and inorganic dissolved nitrogen. As with
phosphorus, the zooxanthellae appear to be nutrient limited
by their host, and here it extends to nitrogen. Particulate
material or zooplankton captured by the coral are available
first to the coral whereas the animal has little control over
the availability of dissolved intracellular nitrogen. Again,
as with phosphorus, the algae are able to control the amount
of nitrogen that they take up, provided it is made available
(as in enriched conditions). However, unlike phosphorus, availability
of dissolved nitrogen to zooxanthellae has a number of important
effects: First, nitrogen made available to the zooxanthellae
is taken up and used to produce proteins and increase the
rate of mitosis. Even slightly elevated nitrogen levels can
quickly result in rapid increases in the density of zooxanthellae
as they use it to fuel their own reproduction. Second, there
is a subsequent drop in the amount of carbon and nitrogen
translocated by the algae to the host. With increased densities,
the zooxanthellae begin to translocate less carbon to their
host, as apparently they need it themselves (Muscatine et
al 1989). It is also somewhat equivocal that corals are able
to utilize nitrate (which exists nearly totally in its ionic
state at physiological pH) at all, and an inability to find
nitrate reductase in many studies makes the ultimate importance
of this dissolved nitrogen source to corals (and anemones)
rather tenuous. However, it is unambiguously true that ammonium
is a sought-after nitrogen source by both coral host and algal
partner.
Carbon
Carbon is rarely discussed in terms of
its limitation on coral reefs. Inorganic carbon is usually
abundant in seawater either as carbon dioxide or as a form
of carbonate. Additionally, dissolved inorganic carbon can
be found, as well as making up components of the many other
sources of dissolved organic material. Furthermore, and at
least for corals living in shallow water environments, the
productivity of the zooxanthellae in providing reduced carbon
compounds often exceeds the needs of both symbiotic partners.
Primary production via photosynthesis, at least in relatively
shallow areas, also tends to have a photosynthesis to respiration
(P:R) rate of greater than 1, meaning that more carbon is
being produced than is being respired. Thus, carbon is the
least of the worries for reef organisms, corals, and us (as
aquarists). The situation may be somewhat different in deeper
water, but algal remains, particulates, and other organic
material usually prevents any shortage of carbon even outside
the photic zone.
Silicon
The role of silicon is much less studied
than other nutrients, however it is considered to play an
important role in the requirements of many coral reefs organisms
such as sponges and diatoms. Their role on corals reefs, especially
since reefs are mostly calcareous based and not siliceous,
is less than other marine environments. The principle source
of silica is likely from terrestrial runoff, and its principle
form, silicic acid, is found in a dissolved form in seawater.
Humic (refractory) compounds
I will only briefly mention humic compounds
because of their interest to aquarists. These yellowing compounds
are typically found at relatively high levels in aquaria,
and they are removed with protein skimming and activated carbon.
Humic materials have been thought to be relatively inert and
unavailable for use as a nutrient source. However, studies
have shown that organisms from bacteria to brine shrimp (Artemia
salina) can utilize humic substances as a source of nutrients.
It does, however, appear that they are less preferentially
utilized than other dissolved organic and inorganic sources.
Bingman has treated humic substances extensively in the aquarium
literature (Bingman, 1996).
Summary
Dissolved organic and inorganic nutrients,
and their role to coral reefs (and corals) is still dramatically
unknown. The complications arising from methodological problems
and poor studies have only compounded the confusion. In terms
of corals reefs, the amounts of most dissolved nutrients,
except carbon in most cases, are very low. Depending on the
location, the reef area, and the organism, various nutrients
can limit the growth of the entity. An example well known
to many is the nutrient limitation of algae. The recycling
nature of the coral-zooxanthellae symbiosis has given a competitive
edge to corals in low nutrient environments, despite the abundance
of highly grazed turfs, unicellular microalgae, and crustose
coralline algae on reefs. However, and as we saw from the
results of excess nitrogen available to zooxanthellae, the
algae are eager to take advantage of increased dissolved nutrients.
Such inputs can lead to algal dominance in the lack of sufficient
grazing.
To aquarists, this means that a "high
nutrient" tank may have algae problems in the lack of
adequate grazing. Put another way, if a tank currently has
a given level of herbivory and a low nutrient content, increasing
dissolved nutrients may allow algae to increase and become
problematic without a subsequent increase in herbivores. Even
so, the higher nutrient levels may cause corals and other
symbiotic partnerships to decline as the partner algae preferentially
utilize the increased nutrient sources to the expense of the
host.
Without question, corals and many reef
organisms are able to utilize dissolved nutrients to help
meet their energy requirements and to use in tissue growth.
However, of all the nutrient sources available, and discussed
in the previous articles in this series, these may be the
least desirable to provide - both in aquariums and to wild
reef communities.
This concludes my series on the "food
of reefs." I do hope it has been enlightening and provides
readers and aquarists with a more complete view of how the
organisms in our tanks acquire the materials they need in
order to grow and thrive in our aquaria. If nothing else,
I hope it serves to emphasize the importance of adequate nutrition
not only for corals, but for our miniature reef tank ecosystems.
I conclude with a quote by Kenneth Sebens that I have used
often in my writings, postings, and presentations to underscore
a very simple point that has, for far too long, been an inappropriate
assumption by too many reefkeepers:
"Corals Are Not
Plants."
Links to
Part
1, Part
2 , Part
3, Part
4, Part
5, Part
6
|

