| Fewer
topics have given me as much consternation over the years than
the subject of bacteria. The microbes are undoubtedly the largest
biomass of life in virtually all ecosystems, and this can be
extrapolated to include the reef aquarium. To most aquarists,
bacteria comprise the “vast unknown,” and perhaps
for good reason. They are vastly unknown, even in nature. However,
aquarists may have some inkling that bacteria are a fairly large
and diverse group of organisms, but generally think of bacteria
in terms of “filtering” water (such as in nitrification
and denitrification) and in terms of the mysterious “bacterial
infections” that seem to rise up to slay fishes and invertebrates
in their tanks.
In this article, I will very briefly use
some examples and analogies to introduce some of the roles
of bacteria in marine environments, and then proceed to their
role as a trophic resource, or food, to corals (and other
reef organisms).
Introduction: Microbial Ecology 101
Bacteria are the oldest living things on
Earth, first appearing 3.5 to 4 billion years ago and were
the only forms of life for about at least a billion years.
Present day life would not exist without bacteria, and these
early bacteria caused global geochemical cycles that made
the Earth habitable and led to the evolution of eukaryotic
organisms. In fact, eukaryotes were able to expand their diversity
by acquiring bacteria as endosymbionts. Interestingly, some
simple protozoa, which consume bacteria and can harbor them
as endosymbionts, resemble the phagocytic cells of higher
organisms, and the bacteria in many ways resemble mitochondria,
the so-called power plants of cells.
To date, no environment on the Earth has
been found to be free of bacteria. They are tiny, and only
a very few species are visible to the unaided eye. Because
of their diversity, relatively few bacteria are well known.
Most of those that are well known either have some relation
to human health (acting as pathogens, or agents of disease),
or are bacterial “guinea pigs” used as models
for other systems. Probably the most common, and often excruciatingly
one-sided, way people are exposed to bacteria is from the
public press or lay media. Stories of “killer”
bacteria, flesh-eating bacteria, infection-causing bacteria,
and others prompt the production of antibiotic drugs, antibiotic
soaps, lotions, and all manner of weaponry to “eliminate”
bacteria.
Relatively few bacteria cause human diseases,
and of those, many are opportunistic pathogens. These species
have not evolved to act as pathogens, but rather can cause
diseases when conditions allow. The vast majority of bacteria
are innocuous and/or beneficial. I guess the way I would like
aquarists to begin to see bacteria are as things that are
always present, everywhere, covering every surface, and are
generally not problematic. A useful analogy might be the conception
of “bristle worms.” For years, aquarists saw bristle
worms as scary and assumed they were to be eliminated. Bristle
worm traps and other devices were marketed and sold to cope
with these “problem” creatures. Eventually, more
people became familiar with the real nature of these worms
and learned that they were not detrimental, but rather beneficial
– with the exception of a very few types that could
be problematic under certain conditions and to certain organisms
– for example, Hermodice carunculata to gorgonians
and soft corals, and the Bobbit Worm to fishes (and divers!).
The same is true with bacteria as with polychaetes, but taken
to the nth degree in terms of numbers and types. There are
lots and lots of innocuous types, lots of good types, and
a few troublesome types that may become apparent under certain
conditions and to certain organisms.
As an example, consider a sterile culture,
or the sterile conditions of an operating room. Anyone who
has tried to culture phytoplankton under sterile conditions
understands how difficult it is to create sterile conditions.
Even surgeons working in operating rooms use multiple techniques
to try and maintain the highest probability of sterility.
Even so, infections occur very often in hospitals, despite
the use of antibiotics, antiseptics, sterile conditions, and
sterilizing sprays. These infections are not due to any single
cause, but rather many such infections (called nosocomial
infections) occur because the patients in a hospital have
immune systems that are compromised to one degree or another.
Bacteria are so omnipresent that creating the nurturing conditions
in a hospital for human life allows the very quick appearance
of bacteria in that environment.
We eat bacteria with every mouthful of
food we eat. We are covered in bacteria. Bacteria line our
mouths, our guts, cover our hair, our clothes. The bacterium,
Escherichia coli, is a species that has allowed for
tremendous advances in all fields of biological sciences as
a model system and is partly responsible for allowing us to
digest and absorb food as the primary commensal component
of our intestinal flora. Yet, most people probably associate
E. coli with the rarer pathogenic strains that cause
disease and have been in countless newspapers headlines. Clearly,
we live surrounded by and enveloped with bacteria all the
time, and they do not cause us disease. In the majority of
cases, diseases occur when conditions in the environment or
with the host allow for disease to occur. The same will be
seen for the marine environment.
Bacteria in the Marine Environment
Although there are certain areas and species
that have attracted some degree of study, marine bacteria
are, in the scheme of things, virtually unknown. Even their
ecological roles are often generalized and assumptions often
based on better-studied terrestrial systems. However, they
are no less numerous than in terrestrial environments, and
are in many cases, and probably most cases, likely more numerous
and important.
It became obvious that the field of marine
microbiology as it relates to understanding coral reefs had
to advance, as best I can parse from the literature, when
several key events and areas of study emerged.
| • |
First, the study of nutrient dynamics, by
necessity, had to include processes mediated by bacteria
in the benthic habitat. |
| • |
Second, the observation and recognition of marine diseases
causing mass mortalities suggested bacteria as potential
candidates and required investigation. |
| • |
Third, the existence of numerous microbial food webs
that encompassed widely varying species groups, from larvae
to large invertebrates, which included bacteria as a significant
link in the chain. |
| • |
Fourth, bacteria were mucking up otherwise good studies
in biology. For example, in coral studies, the outer surface
of mucus is so enriched with bacteria that investigators
who did not flush the bacterial community from the surface
discovered that measurements – such as respiration
- were skewed by the presence of this active and productive
surface flora. |
| • |
Finally, it was becoming difficult to assess and even
to separate the parts from the whole, since bacteria are
practically inseparable from any surface, and there was
beginning to be more and more evidence of highly dynamic
relationships between organisms and bacteria. |
Given the enormous bacterial biomass in
all ecosystems, it should be of little surprise that they
are food for something, if not many things. Bacteria, being
composed of living material, contain a relatively large amount
of nitrogen, an element in very short supply in coral reef
waters. Nitrogen is closely recycled and horded in such environments,
and many organisms may be limited by it. Therefore, attempts
to acquire nitrogen might very well include methods of acquiring
bacteria, an abundant resource.
For years, bacteria were lumped as being
part of particulate organic material (including reef snow)
because it was difficult to quantify the bacterial component,
but possible to quantify the particulates, and it was known
that bacteria coated these surfaces (and every other surface,
for that matter). In other words, it was rather convenient
to deal with them in this way without actually investigating
a vast new unseen and unknown microbial world independently,
especially since 80-99% of them will not grow in cultures
and are quite difficult to study and characterize.
Bacteria as Coral Reef Food
The biomass and productivity of bacteria
on coral reefs are as great as those in nutrient-enriched
(or “eutrophic”) lakes and up to a hundred times
greater than in the open ocean. Planktonic bacteria in coral
reefs areas are dominated by coccoid, rod, and horseshoe shaped
forms from 0.3-0.8?m in size which have filamentous processes
to allow them to absorb and consume dissolved organic molecules.
Many of the bacteria ingested as bacterioplankton are associated
with detrital material and phytoplankton, to which they attach
with adhesin-molecules and cellular attachment processes,
such as fimbriae. Filamentous bacteria tend to predominate
in periphytonic (on the outside of plants) communities, whereas
motile and attached rods tend to form the primary components
of bottom sediment surface layers. Deeper in the sediments,
coccoid and spore-forms predominate. It has also been found
that the diversity and composition of pelagic forms tends
to change as the water column of an area “matures,”
with the highest diversity found in mature nutrient poor (or
“oligotrophic”) waters, such as coral reefs. This
is because many of the diverse forms found there are oligocarbophylic
(thriving in low carbohydrate levels) and do not thrive in
the nutrient concentrations found in eutrophic waters, even
though the total biomass there may be significantly higher.
However, some studies have found that bacterial biomass in
polluted eutrophic waters and sediments may not be any higher
than in pristine areas. Levels can vary over several orders
of magnitude from 5 x 103 to 2 x 106
cells/ml of water. Levels in sediments and detrital pools
can reach 3-10 x 109 cells/gram with a biomass
of 2-5mg/g. The highest levels are reached in detritally-enriched
fine silts (1 x 1010 cells/gram). Bacteria in enriched
sediments are estimated to be found in extraordinarily high
numbers, comprising somewhere between two to five percent
of the total weight or organic material present. This may
be notable below as to why corals “open up and feed”
when the sediments of a tank are stirred. Even more notable
is the efficiency (60-80% efficiency, compared with about
20% for deposit-feeding animals) with which bacteria decompose
organic material and put it into their own biomass. It is
estimated that decomposed organic material can result in 30%
of the newly formed protein in waters from the production
of microbial biomass.
Detrital food chains are found to predominate
in most marine ecosystems, and it has been found that the
bulk of the diet of herbivores, as well as their nutritional
requirements, comes not from direct consumption of phytoplankton
but by the consumption of the adherent periphyton and detrital
material that is enriched by attached microbial communities.
The importance of these components of the diet appears nearly
universal in marine ecosystems. In pelagic plankton, estuarine,
coastal, and coral reef communities, the consumption of these
components encompasses an estimated 60% –90% of the
total energy flow through pelagic and coastal tropical systems,
respectively. Bacterial primary production or photosynthesis
is highest in sediments and can even equal that of the phytoplankton
in pelagic systems. Photosynthetic rates within the sediments
can be equal to or even higher than on those on the reef itself.
In summary for this section, in virtually all studied marine
environments, bacteria are water purifiers, decomposers of
organic material, and a primary source of protein for both
those animals that directly graze on them and those that acquire
them indirectly through secondary consumption.
Bacteria as Coral Food
Given the importance of bacteria as a food
source in marine ecosystems, it might not be surprising to
learn that they are also a primary food source for corals.
It has been found that bacteria alone can supply up to 100%
of both the daily carbon and nitrogen requirements of corals.
All corals studied consume dissolved organic material, bacteria,
and detrital material. This is more than can be said for any
other food source, including zooplankton and light.
Coral consume bacteria in a number
of ways. First, they can use their mucus and well-developed
epithelial cilia to entrap and consume both attached and pelagic
bacteria. Some corals, such as Turbinaria species,
can beat their mucus into webs with their cilia, and these
webs are cast out like a net into the water column to ensnare
particulate material, primarily bacteria. The cilia then pull
the net backwards towards the colony where the polyps consume
the bounty. The amount of nutrition gained by bacteriovory
under normal conditions is unusually high in terms of efficiency
of capture and ingestion, and studies show a range of average
gains from such resources that depend on both the species
and the environment (in terms of the availability of bacteria
in the water column). Table 1 presents some data from Sorokin
(1979, 1991).
Coral
species |
feeding
rate, % of expenditure on metabolism |
| Capnella
sp. |
1 |
| Cladiella
humesi |
1 |
| Sinularia
densa |
1 |
| Sarcophyton
trocheliophorum |
1 |
| Tunicate, Ascidia nigra |
1.6 |
| Rumphella
aggregata |
3 |
| Sponge, Toxidocea violacea |
3.4 |
| Xenia elongata |
4 |
| Lemnalia
rhabdota |
5 |
| Favites
abdita |
5 |
| Hicksonella
princeps |
5 |
| Plexauroides
lenzii |
5 |
| Goniastrea pectinata |
5 |
| Montipora verrucosa |
5.8 |
| Acabaria
nicksoni |
7 |
| Litophyton arboreum |
7 |
| Dendronephthya
gigantean |
7 |
| Zoanthus
sociatus |
8 |
| Isis
hippurus |
9 |
| Bebryce
indica |
9 |
| Merulina ampliata |
10/11 |
| Lobophytum gazellae |
10 |
| Holothurian, Ophiodesoma spectabilis |
10.4 |
| Paralemnalia
clavata |
11 |
| Stylophora pistillata |
11/25 |
| Montipora eryhtrea |
12 |
| Tubastraea sp. |
13 |
| Goniopora sp. |
13 |
| Sinularia sp. |
15 |
| Symphyllia sp. |
15 |
| Mopsella
aurantia |
17 |
| Palythoa caesia |
19 |
| Fungia scutaria |
20 |
| Pocillopora damicornis |
20 |
| Seriatopora hystrix |
22 |
| Fungia actinformis |
22 |
| Acropora hyacinthus |
22 |
| Acropora squamosa |
22 |
| Porites annae |
31/27 |
| Pavona cactus |
41 |
| Hydroid, Pennaria tiarella |
43.5 |
| Tubipora musica |
75 |
| Hydnophora exaesa |
75 |
| Leptastrea transversa |
85/84 |
Bacteria not only provide carbon and nitrogen
for the polyp, but also provide an important source of phosphorous
for the zooxanthellae, in addition to other elements such
as vitamins and iron. In fact, shallow water corals at Heron
Island gained a larger proportion of their metabolic needs
from feeding on bacteria and particulate organic material
than from zooplankton.
There is, however, significantly more to
this story…it just gets better and better!
Corals not only use their mucus to trap
bacteria, but the coral mucus also serves two other purposes.
First, it is an incredibly good growth medium for marine microbes
resulting in and the surface of corals containings bacterial
communities with densities far in excess of the already significantly
high levels in the surrounding waters and benthos. Second,
and; coral mucus is a primary contributor to some kinds of
detritus, where – the particulate aggregates are held
together by coral mucus with levels of bacteria two to five2-5
times higher than in particulates without the presence of
coral mucus. These aggregatesd not only provide food for corals,
but for all manner of particulate filter feeders, a category
– that encompasses directly or indirectly just about
every living animal on the reef. Furthermore, these microaggregates
contain a tiny recycling community where nutrients themselves
are concentrated 2-3 times higher within the aggregates than
in the surrounding water. These energy “packets”
are extraordinarily important, both to individual organisms
and the entire ecology of the reef.
Corals are also able to selectively culture
specific strains and increase the density of bacteria in several
ways. They “farm” bacteria within recesses and
interstitial spaces of their branches and colonies. Reduced
water flow and microenvironmental conditions allow the proliferation
of microbes used as food. They can also change the composition
of their mucus by altering the production of the mucosecretory
cells of the epithelium. The change in mucosal composition
allows for variations in the microbial community on the surface
in that different species and strains are more adept at exploiting
various components of mucus.
Additionally, particulate aggregates are
concentrated in the coral gut or coelenteron and moderated
by the composition and amounts of coelenteric fluid released
into the gastric cavity. This is yet another way to alter,
increase, or vary the types and amounts of bacteria available
as food, and another method of “bacteria farming.”
Lastly, and only sort-of finally, specific
strains of bacteria may be host specific in that species of
corals may associate with one or several bacterial species
in a symbiotic relationships. Increasing evidence points to
the idea that the microbes on the surface are not only acting
directly as food, but are involved in the production of specific
compounds either in limited availability or unavailable by
other means. The bacteria may provide these as “leaky”
fluids resulting from their own metabolism. Additionally,
nitrate reduction or nitrogen fixation is occurring, and the
action of such microbes may provide an important source of
inorganic nitrogen to both coral polyps and zooxanthellae.
The specific associations of coral/bacteria may be related
to the specific requirements of the individual coral species.
There are even some corals, such as some Porites
sp., that harbor bacteria intracellularly, and although it
appears to be a commensal or symbiotic relationship rather
than one of parasitism or pathogenicity, much more work is
required to elucidate the true nature of these internal aggregates.
Summary and Aquarium Considerations:
Bacteria exist in very high diversity
and biomass in the marine environment, and especially on coral
reefs and on coral surfaces. They play critical roles in virtually
all ecological processes that control reefs and are a major
component of food webs. Corals feed on bacteria at levels
and efficiencies that rival all other bacterial consumers
(Figures 1 and 2, adapted from Sorokin 1973). Special adaptations
exist with corals that allow them to be able to supplement
their nutritional requirements with bacteria, directly, and
the products of bacteria, indirectly, and even enhance an
otherwise already abundant resource. Levels of bacteria in
reef aquariums are largely unmeasured; however, they are likely
to be comparable to those found in wild communities. As such,
corals in aquariums are likely deriving a significant amount
of energy from the consumption of bacteria on detrital aggregates,
in the water column, and “farmed” on their surfaces.
Attempts to “sterilize” coral surfaces or tanks
by using prophylactic dips or antiseptics and antibiotics
has the distinct potential to do more harm than good in the
majority of cases. Most aquarists probably have a distinct
impression that “good” and “bad” bacteria
exist, but may not be aware of the relative levels of each,
the importance of the diversity and roles of the microbial
community, or the extent of the biomass involved. A more complete
understanding of the roles and importance of bacterial communities
in aquariums is essential in understanding how reef aquariums
function and to provide for their success.
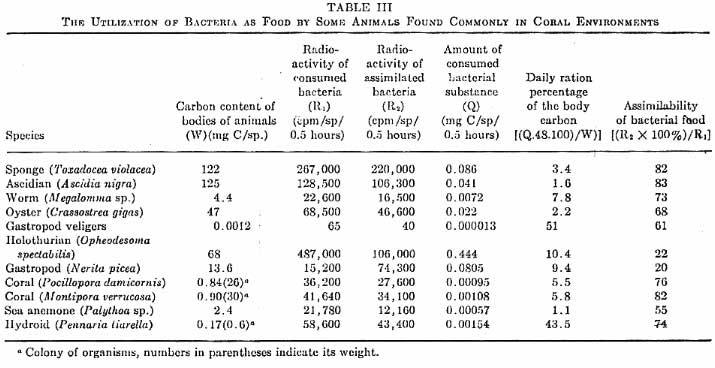 |
|
Figure 1. A comparison
and quantification of bacteria as a food source by various
coral reef organisms. Click on the table for a larger
image.
|
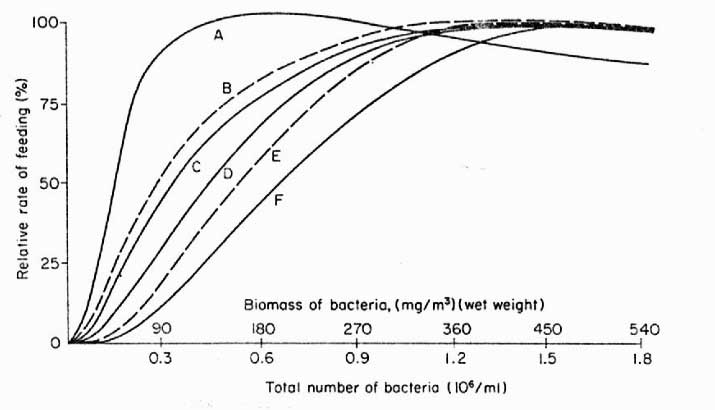 |
|
Figure 2. Dependence
and rate of feeding on bacterioplankton by various filter
feeding animals. A – sponge, Toxadocea
violacea, B – gastropod veligers,
C – serpulid (feather) worms, D
– coral, Pocillopora damicornis, E
– oyster, Crassotrea sp., F –
crustacean, Eucalanus antennatus.
|
Note: I put quite a bit of work
into utilizing and providing references in my articles. I
hope that interested readers will take the time to find these
literature sources, for they provide a much more complete
picture of my article content. In this particular case, some
of the works are absolutely fascinating and simply should
not be missed.
Links to
Part
1, Part
2 , Part
3, Part
4, Part
6, Part
7
|

