|
This is the sixth installment of a seven-part
series on food sources to corals and coral reefs. This article
will address a very important food to corals and many other
animals, particulate organic material (POM). This food source
has many names, including detritus, floculant organic matter,
reef snow, marine snow, suspended organic material (SOM),
and more. While there may be some distinctions between the
material described by these terms, they all basically refer
to the same material.
Not so long ago, marine aquarists made
every attempt to be assured that their water column was "polished."
I never fully understood the term, but the premise was that
a clean water column was a good water column. Various means
were employed to accomplish this, including the use of various
power filters, mechanical flosses and screens, sterilizers,
ozonizers, canister filters, diatom filters, foam fractionators
and many other devices. In this article, I will describe the
composition and role of POM, and discuss why such "polished"
water might not be in the best interest of reef tanks or corals.
What is Particulate Organic Material?
Particulate organic material has its origins
in life, being composed by and large of the remains, secretions
and excretions of living organisms. On coral reefs, it is
composed mostly of dead algae, bacteria, mucus, and feces.
To aquarists, detritus is any flocculent organic material
originating as part of the excess secretions, productions,
breakdown, additions or waste in the aquarium habitat. In
aquariums as in the wild, it has its origin in fish feces,
coral mucus, algal remnants, worm castings and burrowings,
the molts of small crustaceans, uneaten food, and other debris.
Another source of POM to reef environments, depending on the
location, may be organic inputs derived, and potentially composed
largely of terrigenous sediments. Strictly speaking, detritus
tends to settle out of the water column, while suspended organic
material is light enough, as it is nearly neutrally buoyant
to remain afloat in the water column more easily. There is
really no other difference, except that detrital material
that is in suspension can be used by different organisms from
those who could feed on it once settled or incorporated into
sediments.
In aquaria, this material is typically
removed from the water column by protein skimmers or other
filtering devices. Larger heavier particles settle to the
bottom where it forms a fluffy waste that aquarists once found
objectionable, and siphoned it out manually in the days of
bare-bottomed tanks. Live sand soon became a means to biologically
manage detritus, but many still questioned its role as a desirable
component of a captive system. Some early skeptics even proclaimed
it necessary to vacuum, filter, or replace sand periodically
to prevent accumulation of detrital material. Fortunately,
the natural processing abilities of an adequately sized sand
bed seem to be greater than the normal deposition rates of
detritus in all but the most heavily stocked and fed aquaria.
A Paradox
When food, waste, or other particulate
organic matter (POM) is trapped, especially in an aerobic
environment, it is acted upon by several types of bacteria
that break down the substances into more basic dissolved organic
and inorganic components. Some of these breakdown components
are organic acids and refractory compounds that can impart
a yellow tint to the water column. This yellowing has been
called "gelbstoff." Other components of concern
to aquarists are nitrogen and phosphorous, either alone or
complexed to other molecules. These substances typically act
to degrade the water quality in closed systems like aquaria,
and are also partly responsible for the change of many wild
coral reef communities into algal dominated communities. Additionally,
phosphorous (usually as phosphates) can decrease calcification
rates and act as a "fertilizer" for marine flora.
Nitrogen (usually as ammonium or nitrate ions) can increase
the density of coral zooxanthellae, concurrently lowering
amounts of translocated photosynthetic products to the polyp.
Like phosphorous, nitrogen is also a nutrient source for other
flora and fauna. On coral reefs, nitrogen and phosphorous
levels are usually very low, but in aquaria levels can become
problematically high to where the resultant community looks
and functions more like that of a eutrophied reef dominated
by algae.
It is a paradox that in an attempt to "cleanse"
the water column of particulate material using flosses and
other mechanical traps, the result can be poorer quality water
as well as one which is visibly less "clear." To
address the inherent problems of these microbial breakdown
products, activated carbon -often an inseparable component
of filter media - is frequently used in conjunction with some
of these devices in order to remove the yellowing compounds;
the very same compounds that were ironically the result of
the filter media itself. Ozone is also occasionally employed
for this purpose. Following the absorption of some of these
refractory compounds, the result is a variable amount of dissolved
organic matter (DOM) and inorganic nutrients that simply pass
through the filter. However, both the substances remaining
after filtration, as well as the substances removed by filtration,
can be utilized by the life in the aquaria and are taken up
by corals, sponges, some other invertebrates, phytoplankton,
bacteria, and algae.
The products of aerobic bacterial breakdown,
unless accompanied by "the other half" of nutrient
cycles (mineralization, denitrification, and other reductive
and/or oxidative processes, etc. that "regenerate"
nitrogen and phosphorous) can far exceed the amount of direct
uptake by living organisms in many tanks. For this reason,
the use of live sand beds (and other more questionably effective
means such as resins, media, denitrators, powders, etc.) are
often employed to address at least part of the remaining decomposition
and recycling processes that would occur in nature. Protein
skimmers seek to "short circuit" the process partly
by removing particulate matter before it is broken down by
microbial action. Nitrification also typically occurs much
more quickly than denitrification. Unfortunately, most aquarists
do not have a few millennia to spare to allow for complete
remineralization of organic matter or other processes often
measured in centuries, not months. This is especially true
given the much larger area of low-conductivity and low-oxygen
state regenerative spaces in the wild. Therefore, increasing
uptake by various organisms in the aquaria, coupled with both
aquarist aide (water changes, nutrient export devices) and
biological aide (live rock, sand, etc.) are often the best
we can do.
Particulate Organic Material as a Trophic Resource
Both in the aquarium and on coral reefs,
detritus is mostly algal in origin, being produced in situ
and is thus referred to as being autochthonous. It is composed
mainly of dead filamentous algae and phytoplankton, and secondarily
of fleshy macroalgae, coralline algae, zooxanthellae, cyanobacteria,
phytoplankton and seagrasses. Non-algal detritus is mostly
congealed coral mucus bound with other particulate material
(Alongi, 1988). On reef slopes and crests, the material is
mostly coral mucus while over reef flats and lagoons, the
material is mostly algae and fecal matter. This material,
by itself, has a high carbon content. However, it acts as
a substrate for bacteria, ciliates, cyanobacteria, and other
microorganisms that coat the particles. Bacteria can even
convert dissolved organic material (DOM) into particulate
organic material (POM) by aggregating it in the presence of
carbon. This provides a substantially enriched particle replete
with amino acids and valuably higher nitrogen content. As
such, detritus becomes a very nutritious food source for many
organisms. It is such a complex "dirt" that detritus
has been described as a completely self-contained microhabitat
of its own with plant, animal and microbial components and
its own "built-in" nutrient source.
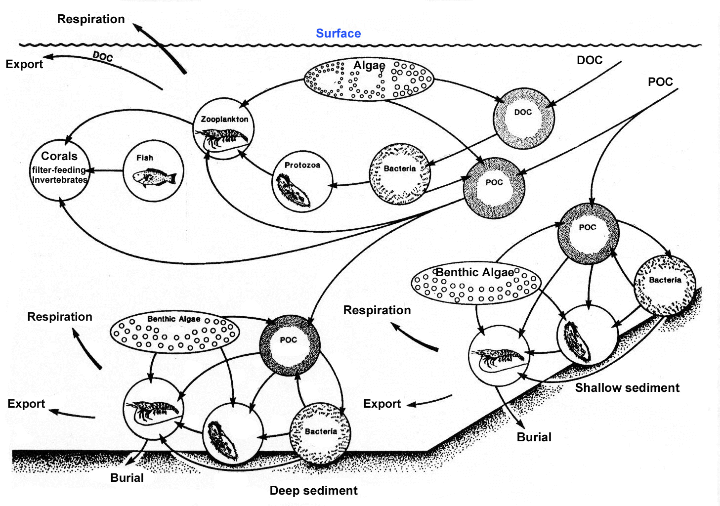
|
The
diagram above shows the cycling of detritus (POM) in
the water column above the sediments. It should be apparent
that nearly the entire food web is dependent on this
material. (Click for larger image).
|
The role of detritus in the food chain
is mostly determined by water velocity and exchange, and the
benthic community (sand flora and fauna). In areas of strong
water flow and exchange, less detritus is deposited and is
flushed away. Aquarists may be familiar with the term "detritivore."
This term encompasses certain animal species known to feed
primarily on detritus and considered to provide a "janitorial"
role in some aquariums. Among such animals commonly utilized
are sea cucumbers, brittle stars, sand dwelling sea stars,
and certain "sand-sifting" gobies such as Valencienna
spp. More recently, some facilities have been providing "detritivore
kits" that include smaller detritus consumers such as
various polychaete worms (bristle worms), amphipods, and small
mysid shrimps.
These are perhaps the more well-known
detritivores to aquarists, but are only a few of the potential
consumers of detritus on a reef or in an aquarium. In fact,
depending on the composition of individual particles, fish
and invertebrates may intentionally or unintentionally consume
and process significant amounts of detritus in their grazing
activities. One study showed that a third of planktivorous
fish had from 1-88% of their gut contents composed of detritus,
and herbivorous fish have similarly high amounts. Crabs and
shrimp can be heavy consumers of detritus. Detritivory occurs
both pelagically (in open water) and in the benthos (substrates
such as sand and limestone framework). On coral reefs, the
major detritus consumers are sea cucumbers (holothurians)
and thallassinid shrimp. Sponges are capable of taking up
small particles, as are many sessile invertebrates such as
sedentary polychaete fanworms, feather duster worms, anemones,
tunicates, crinoids, and other filter feeders. Some intentionally
intercept this particulate organic material while others receive
it by gravitational deposition onto their surfaces. Deposit
and filter feeders typically get from 2-50% of their nutrition
from detritus. Another major consumer group of detritus is
the zooplankton. These small animals, themselves a very important
food sources to reef consumers, have been found to have 90%
of their gut contents composed of detritus. Mucus-producing
animals, like corals, tend to trap detritus, and the material
is either removed or consumed by ciliary action across the
tissue surface. Many fish also consume coral mucus, and any
attached particulate organic material. In some animals, such
as basket stars and crinoids, flocculent material of a certain
size may be a limiting resource to sustain their metabolic
and nutritional needs, and may be at least partly responsible
for their lack of survival in aquaria.
|
|
|
Filter
feeders such as these polychaete worms (feather duster
worms) become very prolific with the availability of
detritus. They reproduce readily in aquariums with a
proper food source. Photo by Eric Borneman.
|
Detritus as an Ecosystem Component
Detritus plays a critical role in the integrity
of coral reefs, and it plays a similarly important role in
the aquarium. There are five fates for detritus in an ecosystem
(captive or wild): 1) Use by the microbial community 2) consumption
by macroconsumers like fish, crustaceans and other detritivores,
3) incorporation and permanent burial in sediments, 4) export,
and 5) regeneration. At least in the wild, and probably in
aquaria, very little detritus reaches the sediment to be buried
and incorporated into it permanently. In coarse sands or rubble
areas, microalgae and cyanobacteria may be the primary site
for uptake of microbial decomposition processing of detritus,
while in finer sands bacterial uptake predominates.
Detritus forms the basis of several food
webs that are part of a balanced autotrophic/heterotrophic
community. It also plays a role in establishing various levels
of nutrient production and decomposition. It is this material
that is the principal food source for the many bacterial species
that work in various nitrification and denitrification activities.
Before reaching the microbial community, however, it acts
as a food source for the smaller consumers such as amphipods,
copepods, errant polychaetes, protozoans, flagellates, ciliates
and other animals whose activities contribute to the stability
and productivity of a coral reef and a coral reef aquarium.
It is the microbial community, though,
that is most important in the detrital processes. On the reef,
the productivity of bacteria (both aerobic and anaerobic oxidation
and reduction, including important sulfate reduction) depends
heavily on detritus. Without this microbial community, coral
reefs would cease to exist. For aquarists, this translates
more simply; without detritus, the denitrification of live
sand ceases to exist. Microbial productivity is greatest where
the levels of organic material are highest. Thus, disturbing
or vacuuming stable sandbeds in aquaria is likely to be counterproductive.
|
|
|
A
healthy sand bed is stratified with numerous burrows
present from worms and small crustaceans. The coloration
can vary greatly within the bed. Here, green colored
cyanobacteria forms a middle layer below the highly
oxic surface and the more anoxic levels below. Photo
by Eric Borneman.
|
Coral reef benthos is thought to be nutrient
limited. The microbial community is not overburdened under
normal circumstances, and lagoons, seagrass beds and the water
column have vast areas that can process the sum excess production
of the reef and terrestrial inputs. A balance is reached under
ordinary circumstances. If the amount of particulate matter
becomes overwhelming, the communities that tend to respond
first are the bacterial communities, followed by the algal
communities. The same is true in the aquarium.
Under ordinary aquarium conditions, the
detritus produced within a tank is easily managed by the various
consumers and microbial flora. In the process, detritus provides
an important food source or food web link for the tank's inhabitants.
Under ordinary circumstances, the microbial community and
various consumers will respond in classic cyclical fashion
to fluxes in detritus present. If detritivory and/or microbial
productivity is limited or reduced, or if there are enough
devices enabled to intentionally remove this particulate matter
from the water and substrate, then there may be a limitation
of detritus available to sustain a productive community. The
most notable signs of such a paucity of organic material are
listed in Table 1. It should be noted that phosphorous and
nitrogen levels in the sand bed are normally extremely high
(200-400 ppm), but these pools are normally maintained and
used by the benthic community with released nutrients being
taken up by life in the water column (Entsch et al., 1983).
This is, however, a point of interest for aquarists; sand
beds, as stated before, should not be disturbed!
|
Levels
of Detrital Material
|
|
Low
|
Normal
|
High
|
Barren
sand bed relatively devoid of burrowing worms, small crustaceans
|
Abundant
burrowing worms, small crustaceans
|
Worms
and small crustaceans usually only near the very top few
mm's of sand
|
|
No
layering present
|
Stratification
of layers
|
Sand
too dark to see layers
|
|
Sand
almost totally white with little coloration except coralline
algae on glass
|
Visible
patches of color (green, red, grey, brown, etc.) from
microbial, cyanobacterial and algal populations
|
Sand
very dark brown to black, coloration usually only in
the top few mm's of sand near the water interface
|
|
"Sterile"
live rock covered almost exclusively by crustose coralline
algae; little or no sponge growth; few filter-feeders
present or growing
|
Live
rock supports coralline and fleshy algae, sponges, tube
worms, and all manner of filter-feeding invertebrates
|
Live
rock covered predominantly by filamentous and/or fleshy
algae and cyanobacteria
|
|
Table
1. Visible signs characterizing aquariums with varying
levels of detritus and organic material present. Note
that these may represent extremes or ideals, and that
a continuum or mix of signs is far more likely to be
present. However, these signs may serve as a gauge for
an individual system.
|
The Role of Particulate Organic Material in Corals
Corals, in particular, are notable for
their consumption of detritus. All corals studied feed to
some degree on POM and coral communities have been found to
remove half of the POM present on some reefs. So prevalent
is this material that it is termed "reef snow" in
the wild. In some cases, detritus may be the primary heterotrophic
resource of corals, depending on the availability of other
types of living plankton. The soft corals (Octocorallia),
zoanthids (Zoantharia), stony corals (Scleractinia), and mushrooms
(Corallimorpharia), all accept detritus as food and can, in
some cases, be provided with over 100% of their carbon and
nitrogen requirements by this resource alone. In particular,
the gorgonians are well studied in this regard. In fact, some
gorgonians seem to rely exclusively on detritus as a captured
food source; rejecting zooplankton and phytoplankton in many
cases - or at least having these other foods comprise an incidental
part of their diet. Part of the reason that the Octocorallia
are so "fond" of detritus is that their tentacles
are well adapted to "sieving," and their nematocysts
are less adept at capturing large or particularly motile prey.
Corals are also notable in that they not only consume detritus,
but also produce it by the bacterial and algal conglomerations
that are trapped and grow on the very mucus they release.
|
|
|
Sponge
growth is a good indication of small particulate foods
in the water column. Here, foraminiferans exist even
within the sponge growth that is not confined to small
areas in crevices on the undersides of rock. Photo by
Eric Borneman.
|
Often in turbid conditions resulting from
the suspension of organic material, light availability becomes
limited. In such cases, corals may need to acquire more energy
from feeding, and this particulate material alone can act
as a food source that can result in up to 50% of the tissue
growth of such corals (Anthony, 1999). Of the many food sources
available to corals and already discussed in this series of
articles, particulate organic material, dissolved organic
material and bacteria are the most universally accepted food
sources across taxa. Often, bacterial ingestion is included
as part of the POM intake because of the degree to which bacteria
coat and increase the nutrition of organic flocs. In other
words, they are often inseparable from each other. Given the
ability of so many corals to consume and utilize this material,
along with its relatively high abundance and ability to provide
up to 100% of corals' carbon and nitrogen requirements, it
may now, hopefully) seem rather foolish to attempt to remove
this material from aquaria.
|
|
|
Because
sediments collect detrital material and are high in
nutrients, they foster the growth of grasses and algae.
These plants can then also uptake nutrients from the
water column, keeping the water nutrient level low and
fostering a healthy reef community. Photo by Eric Borneman.
|
|
|
|
|
|
The
photos above also illustrate variations in a healthy
sand bed community, fostered by the settling of detritus.
The top photo again illustrates the stratification
present in an established sand bed. The bottom
photo shows the variation in communities, with dark
anaerobic areas near the bottom and various microbial,
algal and cyanobacterial mats and areas displaying various
colors. Both are riddled with the burrows of excavating
benthic organisms. Photos by Eric Borneman.
|
Summary
The use of detrital material, or particulate
organic material, as food source is a cornerstone of coral
reef ecology and forms what is well accepted to be the base
of the entire food chain. Coral reefs, being generally nutrient-limited
from outside sources, depend highly on recycling of nutrients
in order to maintain high diversity and productivity. Detritus
forms the foundation of this recycling community. Additionally,
when suspended in the water column as a "reef snow,"
POM becomes an abundant and easily utilized food source to
all manner of filter-feeding invertebrates and is a nearly
universally utilized, nutritious, and important food source
amongst corals.
In aquariums, detritus is produced and
consumed at a considerable rate, and yet the suspended components
are actively removed by all manner of aquarium filtration
devices, including protein skimmers. If suspended material
removal is required in individual cases to maintain water
quality conducive to the survival of aquarium inhabitants,
it is suggested that a facsimile of this material is regularly
provided to the aquarium. Many particulate food sources commercially
available, such as Golden Pearls, VibraGro, crushed flake
foods and Cyclop-Eze can provide a reasonable substitution
for particulate organic material as they tend to remain in
suspension easily. This characteristic is important in providing
access to the food by filter feeding animals, including corals.
As stated in a very early installment of this series, corals
are not plants and must eat to survive. Light alone cannot
and does not provide enough of the substances to maintain
and grow animal tissue. Feeding is essential, and if not by
naturally produced "reef snow" within the aquarium,
by some method involving aquarist effort.
The final part of this series, concerning
dissolved organic material, and a summary of the series, will
be provided here, at Reefkeeping magazine, in an upcoming
issue.
Links to
Part
1, Part
2 , Part
3, Part
4, Part
5, Part
7
|

