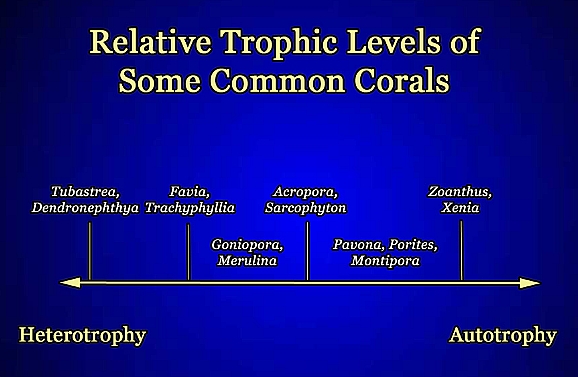
|
Last month, I discussed the topic of food
sources to coral reefs, and this month I will narrow the subject
down to food sources for corals. Coral feeding is part of
a well-orchestrated “three-part harmony,” because corals are
supremely adapted to utilizing all manner of the available
food sources on coral reefs.
The three parts to this story, or harmony, are light,
prey capture, and direct absorption. This month, I will cover only the first part,
the nutritive aspects of light.
The Energy of Light
Before becoming concerned about a repetition
of a bevy of other articles on the subject by many authors,
this will not be a discussion of aspects of lighting, qualities
of light, suggestions for lighting, or anything of that nature.
Perhaps such subjects are interesting; they certainly have
been well discussed, and presumably because of the vital importance
of light to many corals. Rather,
it is my intention here to have the readers understand exactly
why lighting is an important subject in reef aquaria.
As I mentioned in the last article, there
are two basic types of organisms: autotrophs (mostly photosynthetic
organisms) and heterotrophs. Corals are heterotrophs, with
a big caveat. Most reef building corals, or hermatypes, and
many non-reef building corals, or ahermatypes, maintain symbioses
with various dinoflagellate algae called zooxanthellae. While
the coral polyp itself is not autotrophic, its nearly obligate
association with these dinoflagellates provides polyps with
a built-in autotroph that it can, to some degree, control.
Therefore, reef corals with polyps maintaining symbionts
have characteristics of both autotrophs and heterotrophs.
Lighting provides the energy for zooxanthellae to photosynthesize. It may or may not come as some surprise that
light, to corals, is simply food.
Also mentioned in the last article was
the fact that the waters around coral reefs usually have extremely
low levels of various nutrient sources, largely because of
fierce competition for those same nutrients amongst the vast
numbers of species found there. A common and successful strategy
to allow successful competition for habitat space in such
an environment is to utilize an energy resource that is not
generally limited in tropical waters... sunlight.
Corals are not the only organisms to utilize this strategy,
as clams, sponges, hydroids, foraminiferans, nudibranchs,
and many other organisms also host photosynthetic algal or
bacterial cells in their tissues for a similar purpose. As it turns out, sunlight is such a valuable
commodity that means to attain as much of it as possible are
built into the life history strategies and behaviors of organisms
harboring such symbionts. For corals, regulation of the zooxanthellae
population is possible, they expand or contract their tissues
to expose more or less zooxanthellae to sunlight, and they
modify their growth forms to those ideally suited to their
“place in the sun.” Accessory
animal pigments are also produced to further modify the light
environment to which corals are exposed.
Zooxanthellate and Azooxanthellate Corals
Corals far outside tropical areas, or those
in very deep water, do not contain zooxanthellae. Oddly enough,
perhaps, is that there are a great many azooxanthellate corals
existing on coral reefs alongside or nearby their brethren
with symbionts. If harboring zooxanthellae is such a successful
strategy, why don’t all corals have these symbionts? Part of the answer lies in evolution. Perhaps it has not been advantageous for some
species to adopt them, or perhaps not all species have recently
invaded the shallow water zones and have not had enough evolutionary
time to do so.
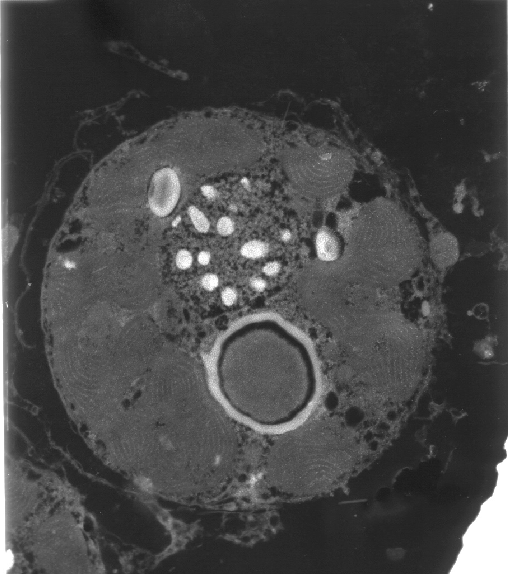 |
| A transmission electron micrograph of
a zooxanthella.The areas with parallel bands are stacked
thylakoid membranes of the chloroplasts where light-harvesting
takes place. |
In fact, there are corals that are facultatively
zooxanthellate; these corals, some from the tropics and some
from sub-tropical regions can exist either with, or without,
zooxanthellae. Commonly researched corals of this type include
some species of Madracis,
Astrangia, and Oculina. In fact, one of the
nemeses of aquarists, Aiptasia
pallida, the glass anemone, is also facultatively zooxanthellate. As it happens, these corals tend to exist with
zooxanthellae in warm, clear, shallow waters and without them
in turbid, cold, or deep waters.
What this means, among other things, is
that if it doesn’t provide much of an advantage to host zooxanthellae
in certain areas, why have them present at all?
It could be argued that some photosynthesis is better
than none at all, even in deep or temperate water.
But, perhaps this is not the case… if there is a cost
involved. And, indeed
there is a cost to the organism to maintain zooxanthellae
within their cells. Apparently,
there is no such thing as a “free lunch” for corals, either.
These facultative hosts must make a metabolic choice
as to whether the benefits of hosting zooxanthellae outweigh
the costs. In most coral reef environments, the symbiosis
is not so optional, and is usually considered to be nearly
an obligate association. I
say nearly, because bleaching is a prime example of when the
costs of maintaining the symbiosis outweigh the benefits,
although the bleaching response is complex and such a statement
represents something of a simplification.
This somewhat answers the question of why
some corals maintain zooxanthellae:
they retain benefits of photosynthesis where prevailing
conditions are advantageous over the costs of maintaining
them, such as on coral reefs. This group includes most of the hermatypic stony
corals, most of the shallow water Caribbean gorgonians, a
couple of shallow water Pacific gorgonians, more than half
of the soft corals, about half of the number of species of
zoanthids (although the vast majority in terms of numbers
of organisms), most of the corallimorphs, and a single genus
of hydrocoral (although this single genus, Millepora,
contains the majority in terms of numbers of organisms on
coral reefs).
It may seem logical that zooxanthellate
species must exist in shallow water to take advantage of sunlight,
but we are still left with one pressing question.
How do some azooxanthellate species seem to compete
so well amongst zooxanthellate species, in particular, some
Pacific soft corals like Dendronephthya species and Tubastraea
micranthus? The
answer lies in the fact that most probably compete well with
their rapid growth, prolific asexual reproduction, and other
behaviors. For the most part, zooxanthellate corals do
indeed compete their azooxanthellate kin for sunlight-drenched
areas, often resigning those without algal partners to recesses,
caves, nooks, crannies, and seemingly less desirable real
estate. Lest it be
thought that azooxanthellate corals are “inferior,” it is
more accurately stated that, like many specialists that exist
on reefs because of their specialization, they compete well
where others cannot. In other words, every organism finds its place
where it tends to be successful.
As testimony to the fact that zooxanthellae are not
necessarily the penultimate adaptation to shallow coral reef
waters, at one time in history, all corals were azooxanthellate
and did not compete for the space to catch sunlight at all.
Types of Zooxanthellae
So successful is the symbiosis between
corals and their zooxanthellae that multiple relationships
have developed. At one time, and not long ago at all, coral
researchers were convinced that all corals held but one type
of symbiont within them. These
single celled algae were called, despite numerous synonymous
names, Symbiodinium
microadriaticum. Eventually,
several other dinoflagellate zooxanthellae were found in the
fire coral, Millepora, and in some zoanthids. However, it was still largely assumed that all
other corals harbored a single species of algae. About twenty
years ago, the walls around such a notion began to crumble,
and it is now recognized that there are many clades (groups
of biological taxa that includes all descendants of a common
ancestor), such as species, types, and subtypes of zooxanthellae
that inhabit coral tissue.
In fact, so widespread is the diversity beginning to
appear that a complete rewriting of coral symbiosis is beginning,
with only a few introductory chapters written as of today.
The diversity and nature of the various relationships is now
hardly known. What is known is that not only may there be
a variety of one coral/one symbiont relationships, but that
various corals may harbor more than one symbiont, may potentially
be able to harbor more than one symbiont even if it is not
usually found to do so, and that even single corals may harbor
more than one symbiont at the same time. I would refer the reader to more information
in my article here,
even though much of that information has already changed,
so rapid are the advances in this field.
There is a very large body of science regarding this
subject, and to cover it in much more depth without many additional
pages, I am afraid, would be doing the subject an injustice.
What The Symbiosis Provides And How Much
Given the general background above, I can
now delve into the crux of this relationship and describe
just what it means to house autotrophs in a heterotrophic
body. Zooxanthellae are initially acquired either from the
water column (in broadcast spawning corals), or are given
a starter culture from the parent polyp (in brooding corals).
Over the course of their lives, coral polyps maintain
various densities of zooxanthellae in their tissues according
to environmental and metabolic conditions.
Polyps periodically release or lose some, require more
from the water column, and control their growth and reproduction
within their tissues quite effectively. For a description of when the symbiosis does
not go quite as smoothly, a process known as coral bleaching,
see this article.
The algae are maintained mostly in the underlying tissue layer,
the gastrodermis, and within the tentacles of some species,
in small containment vesicles called vacuoles. These vacuoles are formed within the gut cavity
of corals after the dinoflagellates have been swallowed, and
they can even migrate across tissue layers.
Once in place, the zooxanthellae
reproduce until they form a mostly single layer within the
tissue; an arrangement that maximizes light capture as a photosynthetic
umbrella, or antenna, while minimizing shading of adjacent
algal cells.
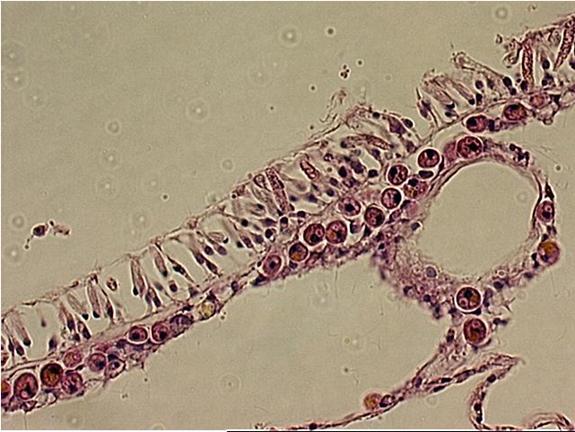 |
| The upper layer of the
Acropora sp. is the epidermis. The lower layer is the gastrodermis. Within
the cells are round to oval golden spheres. These are
the zooxanthellae. |
The zooxanthellae are then carefully controlled
by their coral host by being subjected to nitrogen limitation. As mentioned in last month’s article, nitrogen
levels in coral reef waters are typically extraordinarily
low, with most being found as ammonia.
This is in contrast to aquaria where the dominant nitrogen
species is usually nitrate.
Nitrogen is the end all-be all for zooxanthellae growth
and reproduction. By
limiting nitrogen in the form of excretion products, the polyp
keeps the zooxanthellae in the numbers and density that maximize
photosynthetic efficiency for its own use.
Using several released compounds, most of which are
still unidentified, the polyp stimulates the zooxanthellae
to release virtually all of the products of its photosynthesis,
and these are then used by the polyp for its own needs. If nitrogen was made readily available to the
zooxanthellae (for example, if high levels were present in
the water and the dissolved nitrogen “diffused” into the coral
tissue), it could then be accessed by the algae without limitation
by the polyp, and zooxanthellae could begin to grow and reproduce
like a “phytoplankton culture.” In this case, the symbiosis becomes less advantageous
to the coral, and it will expel some of the symbionts to try
and re-establish maximal benefit from its algal partners. As a practical note, when very high densities
of zooxanthellae exist in coral tissue, the resultant coloration
of the coral is usually a rich or dark brown color.
This relationship may not sound altogether
“symbiotic.” It may even sound parasitic, since the coral
is clearly taking advantage of the zooxanthellae, and seemingly
without much “giving.” Yet,
nitrogen is so limiting on coral reefs that even the limited
excretion of the coral provides a relatively stable supply,
as well as a protected stable environment, to the zooxanthellae.
Given that corals are “squeezing” their
symbionts for all they are worth, what exactly are they worth?
As it turns out, the symbionts provide a constant “sugar
fix.” The high carbon products of photosynthesis are
mostly sugars, and the coral squeezes out almost 100% of the
algal production, allowing just enough to maintain the algae’s
carbon needs for its survival.
In shallow clear water, efficient corals can get over
100% of their daily carbon needs from their zooxanthellae.
These photosynthetically-derived sugars are then used
by the coral for metabolic functions that require energy,
and much of them are lost in the copious production of mucus.
Coral mucus, in turn, and as was shown in the previous
article, is itself a food source to the reef. The production of mucus by corals is also very
important for their protection, food acquisition, competition,
and other functions.
Unfortunately, zooxanthellae don’t make
much else besides sugar. The
coral squeezes out what it can, but not much more ever results.
In particular, nitrogen, once again, is a problem. It seems everyone on the reef is always scrambling
for nitrogen, the substance needed to produce protein; proteins
required for nematocysts, vitamins, tissue maintenance, injury
repair, cell division, growth, gamete production, even the
very toxins used to paralyze prey.Proteins are the ticket
to growth and reproduction in zooxanthellae, as well as for
coral polyps. Thus, it may come as little surprise that this
great sugar fix provided by symbiotic algae comes up rather
nutritionally short in the course of coral nutrition.To survive
and, hopefully, thrive, corals need more than light.They need
to swallow more than their symbiotic zooxanthellae. And this
will be the subject of next month’s article.
Links to
Part
1, Part
3, Part
4, Part
5, Part
6, Part
7
|

