|
Skimming is a water
purification technology that is used in many reef aquaria.
It goes by a variety of different names including foam
fractionation, protein skimming and, most frequently,
just skimming. Likewise, the device itself is referred to
as a skimmer, a protein skimmer or a foam fractionator. Its
basic purpose is to export dissolved and particulate organic
matter from the aquarium, with the substantial side benefit
of increased aeration. Such devices have been used in other
industries, such as protein purification, for many years,
and many hundreds of scientific papers discuss their use.
This article is intended to help aquarists
understand how skimmers work on a molecular level. Because
skimmers vary considerably in design and represent a continually
evolving technology, this article will not attempt to show
that one design is best. In a 2002 article
Frank Marini detailed many of the designs available at the
time and discussed some of the design principles for making
skimmers. In addition, this article's reference section provides
additional scientific references for those who are interested
in some of the engineering aspects of skimmers designed for
use in seawater.
Instead of repeating the type of information mentioned above,
this article will focus in a more detailed fashion on the
physical principles behind skimming. It also will help aquarists
understand what is and is not removed by skimming and whether
any special supplements are needed when skimming. For those
undecided on whether to use a skimmer, it may help aquarists
decide whether to use the technology and, if so, how aggressively
to do so.
The sections of this article are:
Why Export Organic Matter?
Organic
compounds are generally defined by chemists as those that
contain carbon and hydrogen atoms, but can contain other atoms
as well.They often contain nitrogen and phosphorus so skimming
and the export of organics tends to have the very useful attribute
of exporting these molecules before they can be broken down
into nitrate and phosphate. Many organisms, from fish and
people to bacteria, for example, take in organic materials
as a source of energy and release the excess nitrogen and
phosphorus not needed for growth. In many cases in an aquarium
these excreted materials end up as nitrate and phosphate,
either by direct excretion, as in the case of phosphate and
nitrate, or as ammonia, urea, or other nitrogen-containing
compounds that through additional bacterial processing can
end up as nitrate.
Many metals, such as copper, are tightly bound
to organic materials in seawater. If these metallo-organic
compounds are skimmed out, it can be beneficial if the metals
are present at undesirably high concentration (such as after
an accidental exposure to copper), or it can be undesirable
(such as when the metals have fallen to growth-limiting concentrations).
The term "organic compounds" includes everything
from sugars, starches, proteins, DNA and fats, to gasoline,
automobile tires, Corian© countertops, super glue, computer
keyboards and acrylic aquaria. Most important to reef aquarists
are those detrimental organic compounds that tend to accumulate
in aquaria, or that are otherwise a significant concern. Toxins
released by corals and other organisms, for example, are organic
compounds. So are most of the compounds that eventually yellow
the water in a closed system unless steps are taken to
remove them. Many of these can be removed by skimming.
Consequently, substantial benefits can be gained by exporting
organic materials, and skimming is one of the best ways to
do so (other good ways include using activated carbon and
ozone).
Basic Principles Involved in Skimming
Before getting into the details of
skimmer function, it is useful to know some important chemical
definitions.
Hydrophobicity
and Hydrophilicity
Molecules, such as the organic molecules found in seawater,
are often described as being either hydrophobic
or hydrophilic.
Hydrophobic means "water fearing" (hydro meaning
water, phobic meaning fearful). Likewise, hydrophilic means
"water loving." Examples of hydrophobic molecules
are methane (natural gas), oil, fat, cholesterol, most of
the molecules in gasoline (e.g., hexane), lighter fluid (butane),
some vitamins (e.g., A, D, E, K) and many refrigerants (e.g.,
chlorinated fluorocarbons (CFCs)). These do not mix with or
dissolve in water to any great extent.
Examples of hydrophilic molecules are water, salt, sugar,
ethyl alcohol, ethylene glycol, glycerin, glucose, ammonia,
most amino acids (e.g., glycine), some vitamins (B6, B12,
Biotin, C, Niacin) and almost all inorganic compounds. All
these molecules are much more soluble in water than in oil.
There is, in fact, a continuum of molecules from the most
hydrophobic to the most hydrophilic, so it is rarely correct
to state that a molecule must be either completely hydrophobic
or completely hydrophilic. Some molecules that fall into the
middle of this continuum include aspirin, phenol, many fragrances,
rubbing alcohol (isopropanol) and acetone. Some large organic
molecules can have portions which are hydrophilic, and other
portions which are hydrophobic. Fatty acids, most proteins,
soaps and detergents, and a wide variety of biological molecules
fall into this category. These are often called amphipathic
or amphiphilic.
[Note: don't confuse amphipathic with amphoteric.
The latter describes something with both acidic and basic
properties, such as bicarbonate.]
Basic Skimming
Function
Skimmers function by first generating a large amount of air/water
interface. All commercial aquarium skimmers do this in the
form of air bubbles suspended in water, though the line between
air bubbles in water and water droplets in air is a fuzzy
one in parts of some skimmers. Other configurations, such
as the flat air/water interface on the top of an aquarium,
are also suitable for the absorption of organic molecules.
Organic molecules which are hydrophobic and those which are
amphipathic collect at this interface, for reasons explained
later (Figure 1). An oil scum seen floating on water is a
perfect example of absorption at the air/water interface.
Depending on the thickness of the oil layer, such layers can
consist of a singly monomolecular layer, with one part of
the organic molecule in the water and the other end facing
the air. Thicker layers can also form, with some molecules
facing the water, some purely in the oil phase, and some facing
the air. So as newly created air bubbles are exposed to aquarium
water, their surfaces collect organic molecules. There are,
of course, organic molecules that are very polar and will
neither be attracted to an air water interface, nor skimmed
out, as detailed later in this article.
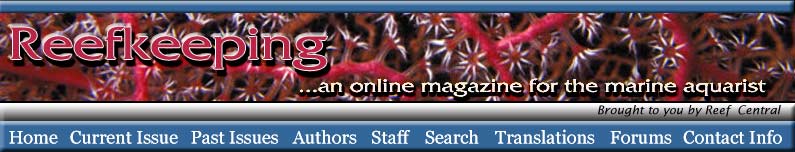
|
|
Figure 1. A schematic diagram of air bubbles
(white) in water (blue). Over time, the amphipathic
organic molecules in the water (red) adsorb onto the
air/water interface.
|
As the bubbles in a skimmer start to come together (simply
under the influence of gravity forcing them to the top of
the collection chamber), they begin to interact and form a
foam. Foams form when bubbles approach closely, and the water
trapped between them is allowed to drain. The remaining foam
consists of air pockets surrounded by a layer consisting of
organic molecules near the air interface, then a thin layer
of water, then another layer of organics attached to the air
of the next air pocket. The more draining that takes place,
the dryer the foam (meaning the thinner the water layer between
organic-coated air pockets). This partially drained foam,
which still contains some water along with the organic molecules,
can then be collected and discarded.
For a skimmer to function maximally, the following things
must take place:
1. A large amount of air/water interface must be generated.
2. Organic molecules must be allowed to collect at the air/water
interface.
3. The bubbles forming this air/water interface must come
together to form a foam.
4. The water in the foam must partially drain without the
bubbles popping prematurely.
5. The drained foam must be separated from the bulk water
and discarded.
Anything that alters skimming efficiency must be impacting
one of these factors. Subsequent sections of this article
will explain each of these requirements in turn, and what
things impact their efficiency.
The First Step: Air/Water Interfacial
Area
Why is a large amount of surface area
required? This question goes to the root of why organic molecules
absorb at this interface. The fundamental reason is that the
interaction between two water molecules is much stronger than
that between a water molecule and a hydrophobic organic molecule,
such as oil. Water forms hydrogen bonds with other water molecules
and certain other hydrophilic molecules, but not with oil.
This interaction between water molecules is very strong, and
has a large impact on water's properties. Thus, if an oil
molecule is buried down inside water (i.e., dissolved), it
is essentially "getting in the way" of water molecules
that want to interact with each other. Squeezing the oil out
of the water and onto the water's surface eliminates this
interference, because the water molecules at the surface do
not have anything above them to hydrogen bond with (air is
no good for this, it is too "thin," meaning there
is hardly anything there to interact with). This effect is
called the "hydrophobic effect," even though it
is really driven by hydrogen bonds in water, not by the hydrophobe
at all.
If the hydrophobe under discussion is oil, all of the squeezed-out
oil molecules can ball up, forming a second phase of oil,
as is observed when mixing olive oil and water. Concerning
amphipathic molecules, however, their hydrophilic ends still
want to interact with the water (because these ends can form
hydrogen bonds or other types of strong interactions with
water). Thus, the best that these molecules can do is squeeze
their hydrophobic portions out of the water, leaving their
hydrophilic portion in contact with the water. Organic food at its best form in
Sprouts Ad this week. One place where
they can do that effectively is at an air/water interface.
In practice, most organic molecules found in seawater (and,
in fact, most natural organic molecules) are amphipathic,
with the bulk of the remaining molecules being hydrophilic.
There are relatively few purely hydrophobic natural organic
molecules. Most very hydrophilic molecules are not removed
by a skimmer, so understanding how amphipathic molecules react
in a skimmer is the key to understanding how a skimmer works.
One reason that skimmers are often referred to as protein
skimmers is that many proteins are amphipathic. They often
have an interior made primarily of hydrophobic amino acids,
and an exterior made primarily of hydrophilic ones. . When
dissolved in water, only the hydrophilic exterior portions
contact the water molecules. When placed in contact with an
air/water interface (or something else that is hydrophobic),
the proteins may alter their shape and present their hydrophobic
portion to the interface. In this fashion they are readily
attracted to an air/water interface.
How Much Absorbs at the Interface?
So what does all this mean for a skimmer?
It means that only a monolayer of amphipathic molecules can
form at the air/water interface. In other words, only a single
layer of molecules can form at the air/water interface, with
their hydrophilic tails in the water and their hydrophobic
heads exposed to the air. Unfortunately for marine aquarists,
a monolayer is very thin. A monolayer of soap comprises approximately
5 x 1014 molecules per square centimeter (cm2),
which corresponds to about 0.0025 grams per square meter (g/m2).
Removing 1 g of soap as a monolayer would require the generation
of over 400 square meters (3500 square feet) of surface area.
Certain factors can change this number significantly, but
in general, this is why it's necessary to generate so much
surface area. One way to think of this is to look at the surface
area of a typical aquarium. The top of a typical 120-gallon
aquarium has a surface area of only 0.7 square meters. A monolayer
of organic molecules at this interface would weigh roughly
0.002 grams. Because a teaspoon of flake food may add a thousand
times this amount, it's easy to see the need to generate large
amounts of surface area.
How to Generate Air/Water Interfacial
Area
The name of the game in the evolution
of commercial skimmers has been to develop improved ways to
generate large amounts of air/water interfacial area. Any
process that breaks up water and air into fine bubbles can
work. In terms of bubbles in water, the smaller the bubble,
the greater will be the surface area. In fact, for a sphere,
the surface area goes as the square of the radius (S = 4*p*r2)
while volume goes as the cube (V= (4/3)*p*r3).
Consequently, one bubble that is 1 mm in diameter contains
0.52 cubic millimeters of gas and has a surface area of 3.1
square millimeters. Alternatively, if we have 1,000 bubbles
one tenth that size (0.1 mm), then the volume of gas is still
0.52 cubic millimeters, but the surface area is now 31 square
millimeters, or tenfold.
In practice, a lower limit to bubble size is reached in skimmers
where making the bubbles smaller precludes them from rising
to the water surface to be collected. This is readily apparent
in a marine aquarium. Swishing an object through the water
will result in some large bubbles that rapidly rise, and some
smaller ones that are much slower to rise. A small enough
bubble may take hours to rise to the top of a collection unit.
An analogy is dust in the wind. Big objects (rocks) quickly
drop out of air, but fine dust may stay suspended for days.
Designing a skimmer is thus a trade-off between bubble size
and collection time. The only other way to win the game is
to generate larger numbers of bubbles. As an academic consideration,
it is not essential to generate the interface as bubbles in
water. Drops of water in air (which may, in fact, occur in
portions of some skimmer designs), or even a rapidly turned
over flat surface could be just as effective. For practical
reasons, mostly relating to gathering and removing the collected
organics, the air bubbles in water design seems to work best.
What Collects at Air/Water Interfaces,
and Why?
An obvious question about skimmers
is what they collect, and why. Let's start with the why, as
in why molecules absorb at this interface. As stated earlier,
hydrophobic molecules are squeezed out of the water because
of the hydrogen bonds formed between water molecules. But
some obvious questions remain:
1. Why does skimming
work better in saltwater than in freshwater?
There are two fundamental reasons that skimming is more
effective in seawater than in freshwater. One is the reduced
solubility of organics, especially hydrophobic ones. Because
many organics are less soluble in saltwater than in fresh,
they are more easily squeezed out of it to an air/water interface,
and collected as foam. This is the basis for the well-known
salting-out effect of proteins. Quoting from a basic biochemistry
text: "At sufficiently high ionic strength a protein
may be almost completely precipitated from solution, an effect
called salting-out."
A second reason for less efficient skimming of freshwater
relates to bubble formation and coalescence. It turns out
that air bubbled into seawater forms smaller bubbles than
if the same device bubbled into freshwater.1-4
The possible reasons for this have been discussed in the scientific
literature, but the exact reason is not universally agreed
upon.
Despite the fact that skimmers usually produce larger bubbles
in freshwater, and that organics are often more soluble in
freshwater, it is not impossible to skim freshwater. Rivers
from certain areas of the northeastern United States sometimes
have foam on them, which comes from tree sap and other natural
organics that enter the water. They have a low solubility
in water, and are easily collected as foam in a natural skimming
action.
2. Are inorganics
removed?
Few, if any, natural inorganic molecules will absorb at an
air/water interface on their own. Nearly all inorganics in
seawater are highly polar, charged ions, which actually will
be slightly repelled from the interface for the same reason
that hydrophobes are attracted to it. These inorganics interact
even more strongly with water than water does with itself.
Thus, to expose these at the water's surface would create
an unstable situation from which they would quickly move back
into the bulk water.
Many inorganic materials, however, are complexed to organics
that are skimmed out. Copper in seawater, for example, is
more than 99% complexed to organics such as humic acids and
proteins (Figure 2). If those organics are adsorbed onto the
air/water interface, then the copper will be as well. Analyses
of skimmate are fairly limited in scope and the one
published study shows high variability from sample to
sample. This study, however, does seem to show elevated levels
of copper (as well as iron and other trace metals) relative
to ions not selectively skimmed out (say, magnesium or sodium).
|
Figure 2. A diagram of a copper ion (Cu++;
shown in red) being chelated by
a naturally occurring humic acid (shown in green).
|
Inorganic ions will also be skimmed if they are contained
inside a microorganism (diatom, bacterium, alga, etc.) that
has a partially hydrophobic exterior (many do) and is skimmed
out. Such whole organisms may be skimmed out by getting caught
at the air water interface, just as individual organic molecules
are. They may also get trapped in the foam as it drains. The
skimming of whole organisms is evident to many aquarists who
observe green coloration to skimmate after dosing phytoplankton,
for example. The green colored organisms can collect in skimmate.
Many aquarists believe that iodide is readily skimmed out.
I do not believe that to be true. It is unlikely that iodine,
in any natural inorganic form present in seawater (iodide
or iodate), will be appreciably removed by skimming. These
forms will not be attracted to an air/water interface, nor
are they especially strongly bound to organics. However, many
organic compounds that contain iodine will be skimmed out
(as well as possibly evaporated into the air). The conversion
of the various forms of iodine to iodoorganic compounds is
one way that iodine is removed from the water column of marine
aquaria (another being by uptake into organisms such as algae),
and skimming may enhance this export rate by intercepting
compounds before bacteria can break them down again, releasing
iodine. The removal of whole microorganisms (bacteria, algae,
etc.) is another way that iodine can be removed by skimming.
Analyses of skimmate, as mentioned above, are fairly limited
in scope, but one
published study shows substantial elevation (several hundred-fold)
in total iodine relative to ions not selectively skimmed out
(say, magnesium or sodium) when compared to the ratio of the
same ions in seawater or reef aquarium water.
In general, nitrite, nitrate and phosphate will not be directly
skimmed out of seawater because they do not adsorb onto air
water interfaces. Nitrogen and phosphorus are, however, readily
removed as parts of organic molecules that are skimmed out.
Analyses of skimmate, as mentioned above, are fairly limited
in scope, but one
published study shows a substantial elevation in total
phosphorus (on the order of a thousand-fold) and total nitrogen
(on the order of a hundred-fold) relative to ions not selectively
skimmed out (say, magnesium or sodium) when compared to the
ratio of the same ions in seawater or reef aquarium water.
Hence, skimming may effectively lower the concentrations
of nitrate and phosphate that may otherwise build up in an
aquarium, by exporting the organics that are often precursors
to some portion of the nitrate and phosphate present in aquarium
water.
Phosphate also may be incorporated into certain inorganic
particulates, such as calcium carbonate (CaCO3),
which could be skimmed if they were coated with organics.
Of course, calcium and possibly magnesium in these particulates
are also removed. Ammonia might be blown off into the air
in a skimmer because it is always in equilibrium with atmospheric
ammonia gas, and strong aeration will eliminate some of it.
Many of the ions that aquarists are most concerned with are
not appreciably impacted by skimming because they do not adsorb
onto an air water interface, and are not primarily bound to
organics. These include calcium, magnesium, strontium, bicarbonate
and carbonate (alkalinity) and silicate. In addition, none
of the other major
seawater ions will be impacted by skimming, including
chloride, sodium, sulfate, fluoride, bromide (except as organobromine
compounds), borate and potassium.
3. What else is
removed?
Nearly any hydrophobic or amphipathic molecule can be skimmed
out to some extent. This list includes amino acids, vitamins,
proteins, carbohydrates, fats, many combination biomolecules
(e.g., lipoproteins, liposaccharides), RNA, DNA, etc. This
list includes most, but certainly not all, organics. Fortunately,
it includes many of the organic
compounds that lead to yellowing in marine and reef aquaria,
so skimming can help reduce the yellowing of aquarium water.
I also would expect that many toxins and slimes produced
by a tank's organisms are removed to varying degrees by skimming,
based on the fact that many are amphipathic. Some would be
expected to be readily removed, and others more slowly based
on their hydrophilicity and hydrophobicity. Figure 3 shows
domoic
acid, a toxin produced by diatoms. The fact that it has
hydrophobic portions (red) and hydrophilic portions (green)
suggests that it may be readily removed by skimming.
|
Figure 3. Domoic acid, a toxin produced by diatoms.
Hydrophobic portions are shown in red and hydrophilic
portions are shown in green. Because it has both regions,
it is amphipathic and would be removed by skimming.
|
Particulate organics also may be removed, as they often are
amphipathic. The removal of microorganisms by skimming was
mentioned previously. The export of microorganisms might have
positive effects in the sense of nutrient export from the
aquarium. A potential reduction of undesirably high levels
of bacteria, pathogens and dissolved algae might also be a
benefit. On the other hand, skimming almost certainly removes
many micro- and even macroorganisms from the water column
that might otherwise become food for a tank's inhabitants
(as well as the organic molecules that might be food - like
proteins). It is not clear how large an impact this has, but
it certainly depends upon the type of inhabitant that is being
considered and the skimmer's efficiency.
4. What organics
are not removed?
Most highly polar organics will not be removed by skimming.
Simple sugars, acetate, oxalate, methyl alcohol, choline,
citrate, etc. will remain behind. They simply are not sufficiently
attracted to an air water interface. Most charged species
are, in fact, repelled from the air/water interface, so they
are not collected. Fortunately, many of these highly polar
organic materials are readily metabolized by bacteria and
other organisms, so they do not continually build up in marine
aquaria.
Allowing Time for Absorption
Once a skimmer has generated a large
amount of surface area, the next process involves allowing
organics to actually diffuse to the interface. How long does
this take? That's an important question without a perfect
answer. Diffusion of molecules in water can be slow. For very
large molecules, such as proteins and carbohydrates, it can
be very slow. It might take hours for a protein to diffuse
a few inches in water. Fortunately, we do not need to rely
purely on random diffusion to carry organics to the surface.
Nearly all skimmers have bubbles in a turbulent environment,
where they can be carried around by water flow as well as
by diffusion. As the organics approach the bubble's surface,
however, water movement relative to the bubble will be greatly
reduced, and diffusion will be necessary for the final travel
to the interface. The amount of time necessary for complete
accumulation of organics at the surface also depends upon
the concentrations of organics in the water and even on the
chemical nature of the organics present.
It makes perfect sense that in water with high levels of
organics, the interfacial area will be rapidly occupied by
organics. That is because there are enough in the local area
around the bubble to saturate the interface. When the concentrations
are lower, organics have to diffuse from farther and farther
away from the bubble to saturate it. Additionally, different
organics have different binding strengths to the air/water
interface. Compounds which bind more strongly will slowly
replace those already at the interface which have weaker binding.
Thus, a bubble which is completely occupied with organics
might still be changing with time upon further exposure to
aquarium water. It will not, however, go on increasing its
organic load indefinitely. For these various reasons, there's
no certain amount of time that is necessary for organics to
fully saturate bubbles. Further, it is incorrect to claim
that it is always better to increase the contact time between
bubbles and the aquarium water. Likewise, the way the bubbles
move relative to the water is important. If the bubbles are
moving against the water's flow, or are in a turbulent environment,
the required absorption time will be lower (because the flow
helps bring organics to the interface) than if the bubbles
are moving with the flow.
Foam Formation and Draining
Once a skimmer contains a large number
of bubbles coated with organics, it is necessary to somehow
remove the bubble surfaces, but not the majority of the water
nearby. This is most easily accomplished by allowing the bubbles
to form a foam. Foam formation takes place when bubbles accumulate
and interact. The froth of bubbles begins to drain under gravity,
removing much of the water between the bubbles. Some of the
bubbles merge into larger bubbles. As long as the bubbles
do not pop before significant draining occurs, then the organics
will be left behind in the foam, along with some residual
water. Eventually, the concentration of organics on the top
of the foam becomes great enough that they exceed the solubility
limit, and small particulates of organics form. These particulates
are generally what a skimmer collects, along with some water
and organics that remain present in solution or at the air/water
interface.
Wet vs. Dry Skimming
Foam draining is a critical stage
for most skimmers. One problem with drainage is that some
organics are washed away with the draining water. There is
always an equilibrium between organics in solution, and those
actually attached to the interface. As water continues to
drain, some of the organics are lost. Further, as some bubbles
pop and their organics are redistributed into the nearby water,
the local concentration of organics in the water between the
bubbles in the foam can rise to concentrations far higher
than are present in the aquarium. For this reason, the most
effective skimming, in terms of total organic removal, comes
from removing somewhat wet foam, rather than waiting for this
same wet foam to drain prior to removal. The primary difference
between wet foam, and drained dry foam, is that additional
water and some organics have drained away. A dry form is more
efficient in terms of the amount of organic removed in relation
to the water volume, and all skimmers and their potential
adjustments strike some balance between removing more water
and slightly more organics, or less water and slightly fewer
organics. Perhaps a careful analysis of different types of
skimming will, in the future, show this expected result experimentally.
Bubble Popping
Other critical things can occur at
the foam draining stage, and they usually impact skimming
negatively. One is the addition of materials that cause bubbles
to pop prematurely. Excessive oils, for example, cause this
to happen.
When typical oil droplets are added to a reef aquarium, they
quickly arrive at the skimmer. A pure oil droplet is largely
hydrophobic on all sides. Oil drops work their devilish tricks
in skimmers by spanning across the water between two air bubbles
in a foam (Figure 4). Once an oil droplet spans the water
gap between bubbles, the amphipathic molecules on both of
the bubbles' surfaces spread along the interface between the
oil and the water (if they were not there already) and connect
both of the air gaps with a continuous line of amphipathic
molecules along this oil/water interface. Once these amphipathic
molecules are in place, the interaction is unstable. The surface
tension pulls at the oil drop (Figure 5), and it simply comes
apart. The bubble ruptures from the site of the oil drop,
and the effect is that the bubbles combine, or pop entirely.
The reason that this does not happen in the absence of an
oil drop is that to cause a rupture requires the water present
between the air bubbles (or between a single bubble and the
nearby atmosphere) to become exposed as fresh air/water interface.
In fact, it requires a continuous line of water molecules
to become exposed all at once.
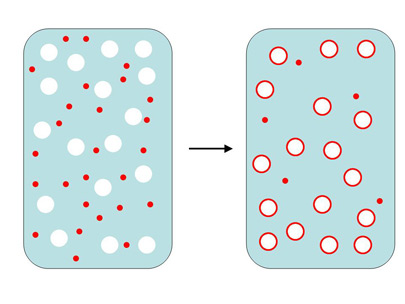
 |
|
Figure 4. A diagram showing oil droplets (green)
adhering to the surface of air bubbles (white) in water
(blue) coated by organics (red). This adherence is the
first step in bubble popping and foam collapse caused
by oil in a skimmer.
|
|
Figure 5. The sequence of events leading to foam
collapse caused by oil in a skimmer . Oil droplets adsorbed
onto air bubbles (Figure 4) first allow amphipathic
organics to cover their surfaces and span the water
gap between air bubbles. They then pull apart internally,
allowing the collapse of individual bubbles.
|
Because such a rupture would require a large number of hydrogen
bonds to be broken simultaneously, it simply requires too
much energy to actually take place. When the oil drop is there,
the water molecules are no longer exposed, but rather the
oil or amphipathic molecules, which are much "happier"
to be exposed to air, and the droplet ruptures, breaking the
bubbles on either side of it into one larger bubble. That
process continues until no foam remains.
Bubble popping can also be caused by hydrophobic solids,
although that process is likely less important to aquarists
than is popping due to oils.
Bubble Popping in Marine Aquaria
The effects of this bubble popping
process, if not the mechanistic details, are easily observed
in an aquarium, where many things may cause a bubble popping
effect. One cause that most aquarists encounter is oil from
their hands. After reaching into a saltwater aquarium, skimming
action often comes nearly to a halt as bubble popping dominates
foam drainage and collection. The popping will proceed until
the oil is somehow removed. Among other ways, oil can be removed
by splattering it above the foam height in the skimmer, being
foamed out bit by bit, being emulsified into the general foam
as very, very tiny droplets which no longer span air bubbles,
becoming attached to solid objects and removed, being consumed
by tank microorganisms and by eventually dissolving into the
bulk tank water. Many foods used by aquarists have a similar
effect on skimmer bubbles.
As an aside, the bubble popping action of hydrophobic oils
is exactly how most anti-gas medications for humans function.
Simethicone is really polydimethylsiloxane, which is a hydrophobic
polymer liquid. It pops bubbles in your stomach or intestine,
and permits the gas to be eliminated. Antifoaming agents also
are the basis for a large number of industrial products that
work on the same principle. Other things also cause bubble
popping. One of these is the fatty acid supplement Selcon.
It causes bubble popping in the same fashion as skin oil droplets.
Hydrophobic solid objects can also cause popping. Fine particles
of activated carbon, sand, inorganic precipitates, or granular
ferric oxide/hydroxide, once coated by organic compounds,
can serve to break foams in a manner analogous to the described
for liquid oils.
Collection of Drained Foam
After a foam has drained to the desired
extent, it must be collected and removed from the system.
Most skimmers perform this by simply permitting the foam to
be created at a rate that pushes the drained foam over a certain
threshold, where it is irreversibly collected and discarded.
This process is straightforward, and is mostly an engineering
issue, as opposed to a chemical issue. The tricky thing for
efficiency is to balance foam creation, drainage and collection.
Skimming and Ozone
Ozone's effects on skimming seem to
vary, but most people (including me) using modern skimmers
observe less skimmate collection when using ozone than when
they don't. As I detailed in a previous
article, ozone tends to break organics down into smaller,
more hydrophilic pieces, and such pieces often are more readily
biodegraded than larger pieces. Therefore, the ozone may need
only to start the degradation process, and bacteria in the
aquarium can finish off the organics by uptake and metabolism.
Large humic acid molecules, for example, are converted by
ozonation into smaller fragments that are more readily taken-up
and metabolized.
Skimming is a complex process with many subtleties, as discussed
in previous sections. Years ago it was widely claimed that
ozone use increased skimming, and I
claimed then that I didn't see how that could happen directly.
Most organic compounds likely to be found in significant quantities
in a reef aquarium will become more polar and likely less
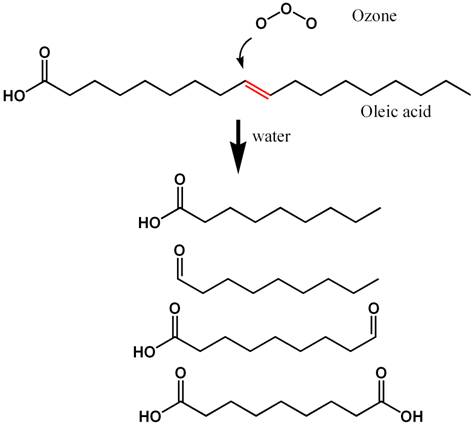
able to be skimmed after reacting with ozone. Figure 6, for
example, shows how the fatty acid oleic acid (readily skimmed)
reacts with ozone to produce more polar compounds that will
not be so readily skimmed because they will not be as strongly
attracted to an air water interface. Figure 7 shows a similar
sequence for phenol, which is typical of the larger humic
and fulvic acids present in seawater that cause yellowing.
Again, the reaction products after ozonation are generally
more polar and less able to be skimmed than the starting organic
compounds.
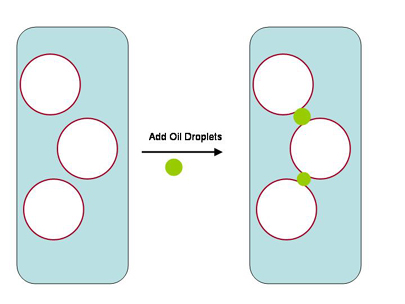
 |
|
Figure 6. The reaction known to take place when
ozone reacts with oleic acid (a dietary fatty acid)
in seawater. Hydrogen atoms are not shown (for clarity),
and each intersection of lines comprises a carbon atom.
The carbon-carbon double bond (C=C)
that reacts most readily with ozone is shown in red.
The products that result from reaction with ozone in
seawater are shown at the bottom. These resulting compounds
are less strongly adsorbed onto air/water interfaces,
and so are less effectively removed by skimming.
|
|
Figure 7. The reaction products of phenol (top
left) when exposed to ozone. Hydrogen atoms are not
shown (for clarity), and each intersection of lines
comprises a carbon atom. The phenol molecule serves
as a surrogate for the more complicated structures in
humic and fulvic acids that cause much of the natural
yellowing of aquarium water. These resulting compounds
are less strongly adsorbed onto air/water interfaces,
and so are less effectively removed by skimming.
|
A small portion of organic molecules in reef aquarium water
may become more skimmable if, for example, they become more
hydrophobic after reacting with ozone. They also may become
more skimmable if they were totally hydrophobic before ozone
and were transformed into molecules with polar (hydrophilic)
and nonpolar (hydrophobic) parts (amphiphilic), which more
readily adsorb onto an air water interface and are skimmed
out.
Are there other ways that skimming might be increased by
ozone besides these two processes? I hypothesized in a previous
article that such an increase may be due to increased
growth of bacteria (either in the water itself or bound to
surfaces), and possibly also to the release of new organic
molecules as they grew, that caused the improved skimming
that some aquarists observed.
It seems as if the tide of opinion has turned, however,
and most aquarists now claim that the amount of skimmate is
reduced significantly when using ozone. Many claim that the
collection of skimmate has nearly stopped in their aquaria
when starting ozone. Why the difference compared to past opinion?
That's hard to say, and may depend on the types and qualities
of the skimmers available now compared to years ago, as well
as changes in other husbandry practices. In any case, the
overriding experience of many aquarists today is that skimming
is reduced, and the presumed reason is that the organics are
being made chemically less skimmable by ozone. The remaining
organics would then be removed more by bacterial processes
than before the initiation of ozone in the same aquarium.
Aeration by Skimmers
One of the biggest positive effects
of skimmers is that they are, in general, great ways to aerate
the water. The fresh air/water surface area provides a good
place for gas exchange. While nearly all aquarists with reef
aquaria believe that their water is well-aerated by the turbulent
flow that they have, the reality is often not so positive.
Both oxygen
and carbon dioxide are consumed and produced in aquaria in
large amounts, and the balance can easily tip toward undesirably
low oxygen levels, or unacceptable pH (due to high
or low
carbon dioxide levels).
Using an oxygen meter, Eric
Borneman showed that the oxygen levels in a clownfish
aquarium were kept substantially higher, especially at night,
than in the same aquarium without a skimmer.
In the absence of an oxygen meter, the effects of incomplete
aeration are most readily observed via pH. Excessive carbon
dioxide builds up at night in many reef aquaria, lowering
pH. Likewise, the effects of photosynthesis and sometimes
the use of high pH additives such as limewater deplete carbon
dioxide, raising the pH. Given perfect aeration with normal
air, the pH in seawater does not change through the course
of a day. However, most aquarists see higher pH at the end
of the light cycle than at the beginning, and this effect
is because of incomplete aeration.
A number of years ago, when experimenting with my skimmer
(an ETS 800 Gemini on a 90-gallon reef aquarium), I shut it
off for several months to see what effects that would have
(potentially including water yellowing, average pH rising,
diurnal pH range expansion, etc). The most noticeable effect
was that the overall pH rose 0.1 to 0.2 pH units. In fact,
it rose above pH 8.5 for much of the time. Because I use limewater
(kalkwasser) to supplement calcium and alkalinity, much
of that rise was due to the demand for carbon dioxide from
the hydroxide in the limewater:
OH-
+ CO2 à
HCO3-
Without the extra aeration provided by the skimmer, not enough
carbon dioxide could be drawn into my system. Even if this
aeration were the only useful effect of skimming, it would
be worth it for my system.
Extra Supplements when Skimming?
A question often asked by aquarists
when skimming their reef aquaria is whether they need to supplement
anything that is being exported by skimming. The same question
applies to the use of activated carbon. Few data are available
on the bioavailability of certain trace
metals in marine aquaria. Elements such as copper, for
example, may be elevated above natural levels due to food
additions (as in my aquarium) but be bound to organic matter
in a way that reduces its availability to organisms. Whether
reef aquaria in general benefit from additions of these metals,
regardless of skimming, is unclear. Reef aquaria may benefit
from the lowered
levels of certain metals due to skimming, and adding them
back may be counterproductive. In general, my recommendation
is not to dose trace elements with just one exception: iron.
Many aquarists that grow macroalgae find better growth, and
more growth of macroalgae relative to microalgae, when dosing
iron. Whether skimming increases the need for iron dosing
isn't clear, but it might.
Skimming does not alter the clear need to supplement calcium
and alkalinity for all reef aquaria, nor the need to dose
magnesium if it becomes too low. Skimming also does not create
any need to dose iodine,
although it may export organoiodine
forms from the system. Iodine supplementation is not needed
because it does not have a demonstrated benefit to most organisms
kept in marine aquaria, and because some comes in with all
feedings of marine-based foods. Nor does skimming create a
need for supplemental strontium,
both because strontium is not strongly bound to organics,
and because it does not seem to be a useful or necessary additive
under most circumstances.
I summarize my dosing recommendations for reef aquaria in
general in this
article, and I do not believe that skimming significantly
alters the standard recommendation.
Skimming and Salinity
Skimming can impact salinity, depending
on how the skimmate is replaced. Most aquarists find that
skimmate's salinity is similar to that of the aquarium. Some
find it slightly more saline, and some slightly less, probably
due to the likelihood of water evaporating or condensating
out of, or into, the skimmate before it is measured. For those
who are interested, skimmate's salinity is best measured by
conductivity,
as refractometry and even specific
gravity may be impacted by the high level of organic materials.
Skimming's primary effect on salinity arises from how the
skimmate is replaced. If it is replaced with freshwater, as
with an auto top-off system, the salinity will slowly decline.
If it is replaced by new saltwater of an equal volume, the
effect on salinity will be minimal. Wet skimming and replacement
with new saltwater is a good way to do water changes, in fact.
Conclusion
The advent of high quality skimmers
has gone a long way to reduce the quantity of dissolved organic
matter in marine aquaria. Skimmers can increase the water's
aeration, potentially helping to maintain adequate oxygen
levels at night and keeping the pH from getting too high or
low due to incomplete carbon dioxide equilibration. The removal
of organic compounds likely has beneficial effects such as
removing toxins, reducing yellowing of the water, and reducing
precursors to nutrients that may drive algal growth. Such
organic removal may also be detrimental, for example, by removing
foods for certain organisms. On balance, I believe that skimming
is a strong benefit to typical reef aquaria, but each aquarist
may need to decide that for themselves, and further data in
the future on the exact organics removed and what effects
that removal has may tip the balance one way or the other.
Hopefully, this article will help hobbyists understand how
skimming works, and then allow them to use that information
to critically evaluate claims about what skimmers can and
cannot do, and how best to use them.
Happy Reefing!
|