Ozone
and the Reef Aquarium, Part 1:
Chemistry and Biochemistry
|
|
Ozone has been used
in reef aquaria for many years. It is claimed to have many
benefits, ranging from increased water clarity to decreased
algae. It has never, however, risen in popularity to the point
where a seeming majority of reef aquarists use it. Many reasons
likely prevent its widespread use, including its cost, complexity
and safety concerns for both the aquarist and the aquarium's
inhabitants. Speaking only for myself, my reasons for never
having used it in my first ten years of maintaining reef aquaria
were driven primarily by concern over ozone byproducts' toxicity
in the aquarium, and the lack of a perceived need.
Back in the early to mid 1990s there was a fair amount of
emphasis on ozone and other oxidizers as a way to raise the
water's ORP
(the oxidation reduction potential). The ORP, in turn, was
incorrectly described as a good way to measure the water's
"cleanliness." So aquarists raised ORP. Then ozone
and other oxidizers (such as permanganate) fell out of favor
for a variety of reasons, not the least of which was the overall
trend toward less technological approaches to reef maintenance.
It appears, however, that the use of ozone may be on the
upswing. In a recent (December 2005) survey I did of experienced
reef aquarists, the results were equally split between those
who had never tried it, and those who were presently using
it or who had in the past and would do so again in an appropriate
aquarium. For most people who had used it, the emphasis is
now on water clarity, not ORP as some surrogate of something
that was vaguely defined but that was supposed to be beneficial.
This article is the first in a series that addresses the
myriad issues around the use of ozone in reef aquaria. The
articles should help aquarists understand why ozone is used
and what molecular level processes take place when using ozone.
Together, they should help aquarists determine for themselves
if ozone is something they want to use, and if so, how to
do so.
The articles are:
Ozone and the Reef Aquarium, Part 1: Chemistry and Biochemistry
Ozone and the Reef Aquarium, Part 2: Equipment and Safety
Ozone and the Reef Aquarium, Part 3: Changes in a Reef Aquarium
upon Initiating Ozone
After a brief introduction to how ozone is used and some
of its claimed benefits, this first article proceeds to describe
what ozone is and how it reacts with seawater. It also relates
ozone's perceived benefits to the actual chemical and biochemical
changes that it can cause. In a sense, it provides the mechanistic
framework for understanding why ozone does what it does, helps
aquarists understand its limitations and details the changes
in the aquarium water that ozone will cause, whether they
are apparent to most aquarists or not (and, in fact, many
are not).
The subsequent articles in this series will address the types
of equipment necessary to effectively and safely use ozone,
and the benefits that accrue upon initiating ozone in an aquarium
system (mine) that had been operating for many years without
it.
The sections of this first article are:
What is Ozone Supposed to Accomplish
in a Reef Aquarium?
I've asked many aquarists what they
believed dosing ozone accomplished in their aquaria. The list
is always headed by increasing water clarity, but also includes
other possibilities. Below, in no particular order, are the
sorts of claims that are made:
1. Increased water clarity (even if it had been very clear
before ozone)
2. Increased light penetration
3. Decreased yellowness
4. Decreased algae
5. Decreased cyanobacteria
6. Decreased skimmate production
7. Increased skimmate production
8. Increased ORP
9. Reduced nitrate
10. Decreased pathogenic bacteria
11. Reduced circulating toxins
12. Cleaner (more pure) water
Some of these make perfect sense, and the chemical and biochemical
mechanisms that cause them through ozone's use will be detailed
in the subsequent sections of this article. Others may not
be correct assertions (decreased pathogenic bacteria, for
example) and these issues are also discussed.
Some instances of apparent problems and perhaps underlying
issues with the use of ozone are subtle enough that most aquarists
never notice them. Bleached corals, for example, are obvious
and have been reported. Perhaps the bleaching that has been
experienced is related to a rapid increase in light penetration.
But suppose that some small invertebrates in the aquarium
were less prone to successfully reproduce due to residual
bromate in the water. Or that the incidence of fish cancers
from bromate (a suspected carcinogen) increased from, say,
1% to 2% for some particular fish species. How many reef aquarists
would notice those changes, or attribute it to the ozone,
even if it were true?
On the other hand, many aquarists might not particularly
care about such subtle issues, and want the water to be clearer
regardless of hypothesized problems. In any case, the data
such as they are will be presented and aquarists can decide
for themselves if ozone use is a practice they want to pursue
or not. At the end of the last article in the series, where
I present the results in my aquarium, I'll comment on whether
I think it is desirable to continue using it or not in my
system.
How is Ozone Used in Reef Aquaria?
How ozone is used will be the primary
topic of the second article in this series, but in order to
understand many of the issues presented in this article, it
is necessary to have a rudimentary understanding of how ozone
is used.
The pathway for ozone entering an aquarium starts with an
ordinary aquarium air pump. The air travels out of the pump
and often into an air dryer. The air's moisture is removed
as it is absorbed by very hygroscopic
solids. Not all aquarists perform this step, but removing
the air's moisture has at least two benefits as the air passes
into the next stage of the process. The next stage is a small
device that generates ozone. The method used by most ozone
generators is to pass the air through a high voltage electric
discharge that breaks apart some of the oxygen (O2)
molecules, and when they recombine, some ozone (O3)
is formed (a second, less effective method uses UV light to
accomplish the same process, either by passing air or the
water itself past a UV light source). Moisture in the air
reduces the amount of ozone formed in the generator, and it
also results in the formation of nitric acid (HNO3;
from water and nitrogen gas in the air). This nitric acid
can reduce pH and alkalinity, and provides nitrate to the
aquarium (which will be discussed in further detail next month).
After the ozone-containing air passes out of the ozone generator,
it usually is sent into some sort of mixing chamber where
aquarium water and the gas are mixed well, and are kept in
contact for at least a few seconds. Aquarists often use skimmers
or specially made ozone reactors for this purpose, and selection
of suitable materials is a concern as the ozone can degrade
some types of plastic, rubber and tubing. The amount of ozone
delivered varies widely. Many manufacturers recommend on the
order of 0.3 to 0.5 mg/hour per gallon of aquarium water,
but many aquarists use less, or do not use it all of the time.
They believe that using less ozone achieves their need for
clearer water, reduces the need for more expensive equipment
and air dryers, reduces concerns about toxicity due to byproducts
and reduces its negative impact on skimming.
Inside the contact chamber, the ozone reacts with many different
chemicals in the seawater including organics, ammonia, iron
and other metals, bromide and iodide. It may also interact
with viruses, bacteria and other organisms drawn into the
chamber. The ozone itself survives for only a few seconds
in seawater, but it leaves other reactive oxidizers (called
ozone produced oxidants, OPO; for example, hypobromous acid,
BrOH) in its wake. These can further react with organics and
other materials and are also potentially toxic, so they should
be removed before the water is released to the aquarium. Much
of ozone's benefits happen in this chamber, where, for example,
the water is made "clearer" as certain pigments
in dissolved and particulate organic molecules are destroyed.
Water leaving the reactor is optimally passed over an amount
of activated carbon sufficient to remove the remaining ozone
produced oxidants. The carbon catalytically (and also noncatalytically)
breaks down these oxidants before they enter the aquarium.
The air passing out of the reactor also contains ozone, and
is also best passed over activated carbon to reduce the aquarist's
concern for airborne ozone's toxicity.
In order to ensure that not too much ozone enters the aquarium,
aquarists should monitor ORP (the oxidation reduction potential)
in the aquarium's water. For those aquarists using a small
amount of ozone, monitoring may be adequate. For those aquarists
using large amounts of ozone, an ORP controller is important.
It can be used to shut off the ozone if the ORP rises above
a set point (that point being either an emergency shut-off
point that is rarely, if ever achieved, or a target ORP where
the generator is actually running only part of the time, and
only when the ORP controller says that ORP needs to be raised
to the set point).
For comparison to other studies reported in this article,
reef aquarists typically use up to about 0.3 ppm ozone in
the "contact chamber" and have contact times on
the order of a few seconds before the water passes into the
aquarium. This value of 0.3 ppm ozone is based on adding ozone
at a rate of 100 mg/hour (a typical addition rate suggested
by ozone generator manufacturers for a tank of about 200 gallons)
to a contact chamber (like a skimmer) that has a flow of 333
L/h; 100 mg/h / 333 L/h = 0.3 mg/L). Higher flow rates, lower
ozone addition rates or incomplete transfer of the ozone into
the water will give lower ozone concentrations in the contact
chamber or skimmer.
All of these aspects of ozone use in reef aquaria will be
explored in more detail next month.
What is Ozone?
Ozone (O3)
is a gas at room temperature, but is not stable enough to
be stored in a bottle. Because it is unstable, aquarists always
generate it on site just before use. The mechanisms for generating
ozone will be detailed in this series' next article, but in
short, ozone is generated by splitting apart oxygen molecules
(O2) from the air and letting them
recombine into ozone.
At low temperatures (below -180° C), ozone can be condensed
to a dark blue liquid. It has a pleasant, sweet odor which
permits aquarists to detect when it is being formed or released,
although it is also potentially toxic. When added to seawater,
it has a very short half life of only a few seconds before
it breaks down.
Ozone consists of three oxygen atoms connected in a bent
line (with an O-O-O angle of ~117° ), while regular diatomic
oxygen consists of two oxygen atoms connected (O2).
Diatomic oxygen is much more chemically stable than ozone.
That is, in part, why ozone is such a strong oxidizing agent.
O2 comprises about 21% (210,000 ppm)
of the atmosphere at sea level, while ozone comprises only
a very tiny fraction (typically about 0.05 ppm).
High in the atmosphere (above about 30 kilometers), light
from the sun breaks apart diatomic oxygen molecules into monatomic
oxygen atoms (O), and that form predominates at all altitudes
above about 150 kilometers. At altitudes between 30 and 90
kilometers, when O is formed, it often collides with an O2
molecule and produces ozone (O3). That
is the atmosphere's "ozone layer." For a variety
of reasons, the actual ozone concentration peaks at about
50 km. It is a strong absorber of UV light with wavelengths
between 200 and 310 nm. It is a far stronger absorber of UV
light than are other gases in the atmosphere. Consequently,
it helps shield the lower atmosphere and the earth's surface
from UV radiation.
Ozone also can be formed in the lower atmosphere and is
generally considered a part of "smog." In this case,
much of the ozone is produced when nitrogen oxides (NO and
NO2) from fossil fuel combustion break
down to release monatomic oxygen (O). As at higher levels
in the atmosphere, this O reacts with O2
to form ozone. Unfortunately, ozone is much less desirable
at lower elevations, where people and other organisms that
breathe it can experience lung damage. When I was a boy growing
up in California's San Fernando Valley, the sky was often
fringed in a brown haze of smog. After vigorous exercise,
my lungs would often hurt when breathing deeply. That effect
is one of elevated ozone's undesirable attributes to humans.
The second article in this series will deal with ozone's
health effects in more detail, but it is worthwhile to show
some basic
information on ozone concentrations here. For
many, the potential undesirable human health effects may be
sufficient to choose to not use ozone in the home for that
reason alone:
Ozone's Effects in the Lower Atmosphere:
0.003 to 0.010 ppm
Lowest levels detected by the average person (by
odor).
0.08 ppm
Latest EPA study (to publish
April 2006) reports significantly increased risk
of premature death in humans. Each 0.01 ppm increase
results in a 0.3 percent increase in early mortality.
0.001 to 0.125 ppm
The natural ozone concentration in air.
0.1 ppm
The typical maximum allowable continuous ozone concentration
in industrial work areas and public and private
spaces.
0.15 to 0.51 ppm
The typical peak concentration in American cities.
0.2 ppm
Prolonged exposure of humans under typical work
conditions produced no apparent effects.
0.3 ppm
The threshold level for nasal and throat irritation.
Some species of plant life show damage.
0.5 ppm
The level at which Los Angeles, California, declares
its Smog Alert No. 1.; can cause nausea and headaches.
1 to 2 ppm
The level at which Los Angeles, California, declares
its Smog Alerts No. 2 (1.00 ppm) and No. 3 (1.50
ppm). Symptoms: headache, pain in the chest and
dryness of the respiratory tract.
1.4 to 5.6 ppm
Causes severe damage to plants.
5 to 25 ppm
Lethal to animals in several hours.
25+ ppm
Likely lethal to humans in one hour.
|
Ozone and ORP
One of the first things that all aquarists
learn about ozone is that it raises the water's oxidation
reduction potential (ORP).
But what does that really mean? In fact, natural seawater's
ORP is a very complex issue, and it is not well established
what has actually changed in seawater when its ORP rises or
falls by a small amount. It may be that the exact ratio of
the more reduced forms of iron and manganese (those being
Fe++ and Mn++)
decreases as ORP is raised, and more oxidized forms (those
being Fe+++, MnO2,
etc) increase.1 Is that
something that aquarists care about? Is it beneficial?
A previous article has detailed the issues around ORP's
measurement and what it means in seawater and reef aquarium
water:
ORP
and the Reef Aquarium
http://www.reefkeeping.com/issues/2003-12/rhf/feature/index.php
Aside from water's exact chemical properties that lead to
ORP, ORP is an indicator of the balance of oxidation and reduction
reactions taking place in seawater. Many of those reactions
will be strongly influenced by adding a strong oxidizer such
as ozone and its chemical byproducts (bromate, hypobromite,
etc.). In that sense, determining the aquarium's ORP level
is useful to aquarists using ozone to ensure that they do
not overdose the ozone.
With sufficient ozone addition, the water will be filled
with highly oxidizing chemical species and the aquarium's
inhabitants themselves will begin to be oxidized by these
species in the water. At high enough levels, these processes
will kill organisms, and it has done so in significant overdoses.
Many aquarists choose to use a particular ORP value as a target
for the amount of ozone to add. In my opinion, the most important
way to use ORP is to stop the ozone addition if the ORP rises
too much. In part this opinion is based on the lack of a direct
relationship between the water's "quality" and the
ORP itself when using a chemical oxidizer. There is, however,
a clear relationship between excessive ORP (say, above 500
mV) and harm to organisms.
Fortunately for aquarists, many of ozone's benefits, such
as increased water clarity and decreased yellowness, can be
attained without the ORP reaching excessive values. Often
the water can become visibly clearer (to the point where the
aquarist simply no longer notices the water in a normal sized
aquarium) with the ORP hardly above 300 mV. On the other hand,
whether undesirably high levels of certain ozone byproducts
are in the water at those acceptable ORP levels has not typically
been studied. What information exists will be detailed in
subsequent sections of this article. The next article in this
series will expand significantly on how to use ORP with ozone
in reef aquaria.
What Happens to Natural Ozone
in Natural Seawater?
Ozone is not significantly generated
in the ocean, but it does get deposited into the ocean from
the air. At the low concentrations of ozone that get deposited
that way, and at the natural concentrations of iodide
usually present in seawater (much higher than ozone), the
ozone can react with the iodide present with a half time of
less than a tenth of a second.2
In this example, the iodide is oxidized to hypoiodate (IO-)
and hypoiodous acid (HOI):
O3
+ I- à
IO- + O2
IO- + H2O
à
IOH + OH-
Because hypoiodous acid's pKa (in freshwater) is 10.4, it
is largely in the protonated (uncharged form) in seawater.3
The hypoiodous acid is itself a strong oxidizer and can go
on to react with other organic or inorganic materials.4
It has also been suggested that very low levels of molecular
iodine (I2) may be generated in this
way in a thin layer on the ocean's surface (0.0002 ppm, or
0.3% of the total iodine).5
One of this reaction's implications is that the use of ozone
will skew a reef aquarium's iodine
speciation, and this is detailed in the next section.
What Happens When Ozone is Applied
to Seawater?
Halogens
When ozone is applied in seawater in concentrations higher
than are naturally present, a larger variety of chemical reactions
take place. Chief among these is oxidation of bromide to hypobromite:6,7
O3
+ Br- à
BrO- + O2
BrO-
+ H2O à
BrOH + OH-
The first reaction is very fast, and the half life of unreacted
ozone in water with a lot of bromide (such as seawater) is
on the order of a few seconds.8
Because hypobromous acid's pKa (in freshwater) is about 9,
it is primarily in the protonated (uncharged form) in seawater,
but a significant amount of BrO-
is also present.3 The hypobromous
acid is itself a strong oxidizer and can rapidly oxidize other
organic or inorganic materials.4
The hypobromous acid can also react in a variety of ways
(including disproportionation and additional oxidation with
ozone) to form bromate:
BrOH
à
à
à
BrO3-
The hypobromous acid can also be catalytically broken down
by ozone to return to bromide:
BrOH
+ O3 à
2O2
+ Br- + H+
About extensive ozonation of seawater, one group concluded:
"Ozonization of seawater oxidizes bromide ion to Br
(hypobromous acid and hypobromite ion) and then to bromate.
If seawater is ozonized for >60 min, essentially all
bromide is converted to bromate."9
That level of ozonation, however, is far more than would
take place in a reef aquarium. The various reactions leading
to bromine-containing byproducts of water's ozonation have
been extensively studied (especially in the context of disinfecting
fresh drinking water that contains bromide). Nevertheless,
it is a complex problem. One recent review3
stated:
"Because bromate formation during ozonation of bromide-containing
waters is a highly non-linear process, kinetic modeling
has been applied to improve mechanistic understanding and
to predict bromate formation. The full model consists of
more than 50 coupled kinetic equations which can be solved
simultaneously with a computer code…"
and then went on to say,
"the predictive capabilities of such models for the
ozonation of any water should not be overestimated."
Well, we won't try to calculate what happens in reef aquaria,
but we will conclude that bromate and hypobromite may be significant.
Bromate is typically the longest lived after ozonation of
bromide-containing water. It is, in fact, one of the biggest
concerns with ozonation as a purification method for drinking
water, because bromate
is a suspected carcinogen. For this reason, the US
EPA limits it to only 10 ppb in drinking water. So in
considering the properties of the treated seawater in aquaria,
both BrOH/BrO- and BrO3-
must be considered.
There is at least one study in the literature of bromate
in a seawater aquarium.10
Here the ozone was used for disinfection, so the doses used
may be higher than many aquarists employ. I also do not know
whether or how effectively they treated the post ozone water
with activated carbon. Nevertheless, the bromate levels in
the Living
Seas exhibit at Walt Disney World's Epcot Center were
tracked. The researchers studying this display found that
bromate had risen to about 0.6 ppm (with nitrate at about
600 ppm). After adding a batch denitrifying system, the bromate
and nitrate concentrations began to drop, suggesting a sink
for bromate that might well exist in many reef aquaria as
well (that is, in systems or locations where denitrification
takes place).
The same reactive pathways that lead hypobromous acid to
bromate will take hypoiodous acid to iodate.
IOH
à
à
à
IO3-
In the ocean, iodine's predominate
form is iodate (IO3-)
with a smaller but significant fraction of iodide (I-).
These two forms' bioavailability to macroalgae and other organisms
varies from species to species, but iodide is often more
bioavailable than iodate. Regardless, the use of ozone
will likely skew the fraction of total iodine toward iodate
and away from iodide. That may or may not be important for
reef aquarists, because the importance of iodine's availability
from the water column to organisms kept in reef aquaria is
undemonstrated, but it may have strong implications if test
kits are used detect some species and not others.
This concern was studied by one group in the Smithsonian
National Zoological Park's Department of Animal Health.11
It claimed that fish need iodide in the water column in the
form of iodide to make the hormone thyroxine. Regardless of
whether that is true or not (that is, whether fish need iodine
in the water or whether they can get it from food), they showed
that seawater's ozonation to an ORP of 400 mV (equivalent,
they claim, to the level attained by skimmer driven use of
ozone) reduced the iodide concentration by more than half.
Ozonation also decreased the concentration of organoiodine
compounds, and raised iodate levels. In the aquarium itself,
iodide and organoiodine compounds were not detectable when
using ozone. They go on to suggest that iodide supplements
might be beneficial in cases when ozone is used. Therefore
the conclusion that "iodine is an unnecessary additive
for reef aquaria," when that conclusion is based
on success in aquaria not using ozone, may not extend to aquaria
that heavily employ ozone.
As long as bromide remains in the seawater, the equivalent
reaction of ozone with chloride
O3
+ Cl- à
ClO- + O2
is unlikely to be significant as it is much slower than
reaction with bromide. The small amount of ClO-
that may form can react with bromide to form BrO-.3,6,8
Ammonia
Another of ozone's potential reactions and its byproducts
with inorganic compounds in seawater is with ammonia. In fact,
ozone is quite effective at converting ammonia into nitrate.
The reaction is fast enough that if sufficient ammonia is
present in seawater, it will happen preferentially to reactions
that lead to bromate.3,12,13
An intermediate species in the process is bromamine (the bromine
equivalent of chloramine),
but fortunately (because it is toxic) it usually is further
oxidized to bromide and nitrate.
BrOH
+ NH3 à
NH2Br
NH2Br
+ O3
+ 2OH-
à
NO3-
+ Br- + 2H2O
Presumably it is not harmful, and may be beneficial to reduce
the ammonia to nitrate more rapidly. It may lead to higher
nitrate
concentrations in the aquarium, however, and may also lead
to a different ratio of nitrogen export via different mechanisms
because some methods (such as growing some species of macroalgae)
prefer ammonia over nitrate.
Iron
Iron
can be present in two primary forms in seawater: ferric ion
(Fe+++) and ferrous ion
(Fe++). Ferric ion is the
more stable form in oxygenated seawater, but ferrous forms
may remain for a substantial period before being oxidized
to ferric ion. The ferrous form is more readily taken up by
many organisms (including people), partly because it is more
soluble and partly due to biological membrane transport mechanisms.
But many organisms can convert ferric ion into ferrous ion
on their surfaces just as they are taking it up, so the importance
of the exact form is not entirely clear. I dose ferrous ion
when adding iron to my aquarium.
Ozone can readily convert ferrous ion into ferric ion.14-16
That oxidation may, in fact, be part of what is actually measured
in seawater's ORP changes. The conversion may be even faster
for complexed ferrous ion than for free ferrous ions in seawater,
and the complexing to organics may be able to keep the ferric
iron in solution even after oxidation.17
Finally, ozone may serve to break iron free from very strong
complexes in which it is not readily bioavailable. Iron
EDTA complexes, for example, may require photolytic cleavage
to become bioavailable in aquaria without ozone, and oxidation
of the complex with ozone may serve a similar purpose.
Oxidation of Organics by Ozone:
Decoloration
The oxidation of organics is, it turns
out, the primary reason that reef aquarists use ozone because
it is the organic material in seawater that causes clarity
and color issues. Its impact on organic materials is also
why ozonation impacts skimming. While most organic compounds
that are exposed to enough ozone for a long enough period
will be oxidized in some way, some are very much more sensitive
than others. In fact, at the levels of ozone attained in a
typical reef aquarium contact chamber (less than about 0.3
ppm ozone) or even disinfection applications where the doses
are much higher, the total dissolved carbon does not appreciably
change during the ozone exposure (although it may later if
bacteria find the newly oxidized organics more bioavailable;
see below).
In a marine mammal pool,18
for example, it was found that disinfection with 4 ppm ozone
with a 30 minute contact time (a disinfection level much higher
than is typically used in reef aquaria) did not reduce the
pool's total organic carbon (TOC) (~13 ppm TOC), while the
use of granular activated carbon (GAC) did reduce it by 37%.
Interestingly, the combination of ozone and GAC was even more
effective, removing 60-78% of the TOC, suggesting that the
ozonation may have altered some of the molecules in a way
that made them bind more strongly (or more rapidly) to GAC.
An alternative explanation that cannot be ruled out involves
biological transformations of the organic compounds taking
place on the GAC surface as it became colonized with bacteria).
One research group19 studying
the reaction between a variety of organic compounds and ozone
concluded:
"…comparisons of rate constants with chemical
structures of the reacting groups show that all reactions
of O3
are highly selective…"
Fortunately, many of the organic compounds that are most
reactive with ozone coincidently are those that aquarists
want to eliminate from aquaria. As seawater ages in marine
aquaria, the water often becomes yellow as a wide variety
of different organic pigments build up. Because of the ozone's
reaction with many natural pigments, it is often used in drinking
water purification for the purpose of "decoloration;"
not organic removal per se, but decoloration.20
In order to understand this effect, it is first instructive
to understand which organic molecules lead to coloration,
because not all of them do. In fact, most organic molecules
are not colored. That is, they do not absorb visible light.
Looking through bottles of purified organic compounds, the
vast majority are white powders. Organisms, however, have
a significant need to absorb light, for example, to photosynthesize
or to see.
In order to generate molecules that absorb visible light,
natural systems often turn to conjugated carbon-carbon double
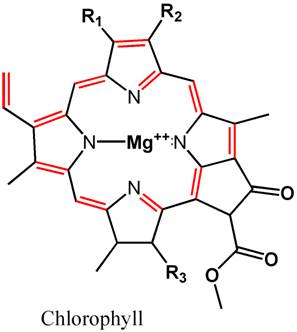
bonds. Figures 1 and 2, for example, show the structures of
chlorophyll and b-carotene. Both
of these molecules are widespread in organisms, and both contain
conjugated double bonds that lead to the absorption of visible
light. These figures do not show the hydrogen atoms (there
are dozens of them), but all of the other atoms are shown,
and there is a carbon at each intersection of two or more
lines. This is how chemists often show structures, allowing
the important features to stand out and not get lost in a
clutter of atomic letters. What is important here is each
segment with a C═C
(shown in red). Without going into ridiculous chemical
detail for a reef article, having a bunch of C═C
bonds arranged together with a single C─C
bond between them can lead to the absorption of visible light.
That is why organisms have developed such chemical structures
for the absorption of light despite their instability toward
oxidation (see below).
 |
|
Figure 1. The chemical structure of the natural
pigment chlorophyll. Hydrogen atoms are not shown (for
clarity), and each intersection of lines comprises a
carbon atom. The repeated carbon-carbon double bonds,
C=C, that are responsible
for absorbing light are also the portions of the molecule
that are most reactive with ozone. They are shown in
red.
|
|
Figure 2. The chemical structure of the natural
pigment b-carotene. Hydrogen
atoms are not shown (for clarity), and each intersection
of lines comprises a carbon atom. The repeated carbon-carbon
double bonds, C=C, that
are responsible for absorbing light are also the portions
of the molecule that are most reactive with ozone. They
are shown in red.
|
It is just that instability, however, that aquarists take
advantage of when employing ozone. Figure 3 shows, for example,
where ozone first attacks oleic acid (a dietary fatty acid).21,22
It is attacked at its double bond, breaking it apart into
smaller fragments that no longer have a C═C
bond. Consequently, while a huge dose of ozone lasting a very
long time will break down these bits even more, even a small
dose will remove the C═C
bond.
|
Figure 3. The reaction known to take place when
ozone reacts with oleic acid (a dietary fatty acid)
in seawater. Hydrogen atoms are not shown (for clarity),
and each intersection of lines comprises a carbon atom.
The carbon-carbon double bond (C=C)
that reacts with ozone is shown in red. The products
that result from reaction with ozone in seawater are
shown at the bottom.
|
Translating that reactivity to the pigments shown in Figures
1 and 2 makes it apparent why ozone is so good at reducing
seawater's coloration and increasing its clarity: it reasonably
selectively targets many of the structures that nature uses
to absorb light, and converts them to nonabsorbing chemical
structures.
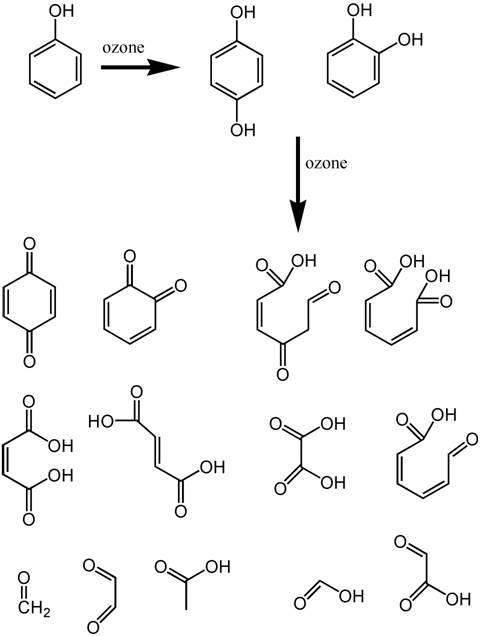
A second type of colored organic compound that accumulates
in seawater (in both the ocean and aquaria) is one of the
functional groups in humic and fulvic acids (the compounds
often identified as the yellowing agents in aquaria).20
These "compounds" are complex mixtures of many compounds,
but among them is the phenol functional group (Figure 4).
Phenol can be attacked by ozone,23,26
with breakdown products shown in Figure 4. It is the Ring-OH
group that is colored when in the Ring-O-
ionized form, and many of these breakdown products lack such
a functional group. Hence the oxidation of such phenolates
in humic acids with ozone will reduce color in aquarium water.
 |
|
Figure 4. The reaction products of phenol (top
left) when exposed to ozone. Hydrogen atoms are not
shown (for clarity), and each intersection of lines
comprises a carbon atom The phenol molecule serves as
a surrogate for the more complicated structures in humic
and fulvic acids that provide much of the natural yellowing
of aquarium water. The light absorbing parts of these
molecules usually involve compounds where OH is attached
to a complete ring of six carbon atoms. Breakdown of
these molecules to bits without a complete ring will
reduce or eliminate the absorption of visible light.
|
The various chemical products described in this section are,
of course, not the only reaction products of ozone, hypobromous
acid and hypobromite with organic compounds. Other products
include brominated organic compounds and many other chemical
structures. These have not been fully elucidated, a fact which
is not surprising since even in the absence of ozone, the
nature of all of the organics
in natural seawater or reef aquarium water remains poorly
defined.
Oxidation of Organics by Ozone:
Skimming and Nutrients
Another result of breaking some organics
into smaller, more hydrophilic bits (Figure 3 and 4) is that
it often increases their bacterial biodegradability.27-29
Therefore, the ozone may need only to start the degradation
process, and bacteria in the aquarium can finish off the organics
by uptake and metabolism. Large humic acid molecules, for
example, are converted by ozonation into smaller fragments
that are more readily taken up and metabolized.29
This process may, in fact, be why some aquarists report drops
in nutrient levels after initiating ozone. It is not because
ozone directly impacts either nitrate or phosphate (it does
not react directly with either), but the newly bioavailable
organics may drive bacterial growth, just as adding ethanol
(e.g., vodka) or sugar might. The growing bacteria need nitrogen
and phosphate, and if they satisfy those needs by taking up
nitrate and phosphate, the levels of those nutrients in the
water may drop. That effect, however, may be only temporary
as the initial burst of new bioavailable organics winds down,
and a new stable state is reached with lower levels of organic
material and similar levels of inorganic nutrients.
Skimming
is a complex process that has many subtleties. The previous
sections have discussed how ozonation modifies organic molecules
, and we can then extrapolate how those processes impact skimming.
Years ago it was widely claimed that ozone use increased skimming,
and I
claimed then that I didn't see how that could happen directly.
Most organic compounds likely to be found in significant quantities
in a reef aquarium will become more polar and likely less
skimmable after it reacts with ozone. Figure 3, for example,
shows how oleic acid (readily skimmed) gets converted into
more polar compounds that will not be so readily skimmed as
they will not be as strongly attracted to an air water interface.
A small portion of organic molecules in reef aquarium water
may become more skimmable if, for example, they become more
hydrophobic after reaction with ozone. They may also become
more skimmable if they were totally hydrophobic before ozone
and were transformed into molecules with polar and nonpolar
parts (called amphiphilic) which more readily absorb onto
an air water interface and are skimmed out.
Are there other ways that skimming might be increased besides
these two processes? I hypothesized in a previous article
that it was due to the growth of bacteria (either in the water
itself, or bound to surfaces), and possibly also the release
of new organic molecules as they grew, that caused the effects
some aquarists observed.
It seems as if the tide of opinion has turned, however, and
most aquarists now claim that the amount of skimmate is reduced
significantly when using ozone. Many claim that the collection
of skimmate has nearly stopped in their aquaria when starting
ozone. Why the difference compared to past opinion? That's
hard to say, and may depend on the types and qualities of
the skimmers available now compared to years ago, as well
as changes in other husbandry practices. In any case, the
overriding experience of many aquarists today is that skimming
is reduced, and the presumed reason is that the organics are
being made chemically less skimmable by ozone. The remaining
organics would then be removed more by bacterial processes
than before the initiation of ozone in the same aquarium.
Ozone and Problem Algae
Many aquarists report a reduction
in problem algae when initiating ozone, although it is not
universally observed. Whether it happens in my aquarium is
one of the observations that I will report in the third article
in this series. However, more people report it than I would
expect if it were a simple placebo effect, where new users
might be looking for a decrease in algae, so they "see"
it. How might algae be decreased? The answer is not clear
at all. No clear explanations were provided to me when I asked
very experienced chemists who have used ozone in aquaria for
many years. Nevertheless, this section provides some potential
causes.
As described above, ozone breaks large organic molecules
down into more bioavailable fragments. Perhaps using ozone
to drive that process increases the rate of bacteria growth
in the aquarium, and the bacterial growth consumes nutrients
just as happens when aquarists dose organic carbon sources
to aquaria to drive bacteria. This process would be related
to the decrease in skimming, where organic molecules are no
longer as effectively skimmed out. Where would they go? Into
the hungry mouths of bacteria that then multiply faster, and
consume nitrate and phosphate in order to produce the biomolecules
of life (proteins, DNA, RNA, phospholipids, etc.).
Another, vaguer, explanation has to do with the ORP itself.
It has been suggested that increased ORP hampers the growth
of microalgae relative to macroalgae and other organisms that
aquarists maintain. There may be a bit of the chicken vs.
the egg argument here, where it is not clear if the lower
ORP drives the algae (by altering the availability of metals
such as iron, for example), or if the algae drives a lower
ORP (by releasing large amounts of organic molecules, for
example). In any case, raising the ORP may well alter the
bioavailability of important metals such as iron. In fact,
even without raising ORP, ozone may break down strong metal/organic
complexes, increasing the bioavailability of the metal. In
either case, ozone may tip the delicate balance of nutrient
flow away from microalgae and toward other organisms (macroalgae,
bacteria, corals, etc).
Ozone Reduction of Organic Toxins
in the Water
In addition to the water's decoloration,
another potential benefit of the ozone's reaction with aquarium
water is the destruction of organic toxins. Many marine creatures
secrete toxins that are designed to be harmful to other organisms.
If these are allowed to build up in aquaria, they might become
stressful for certain organisms. In addition to using activated
carbon and skimming to remove them, ozone may also play a
useful role.
As discussed above, ozone's reaction with organic molecules
involves fairly specific types of reactions, and it does not
remove all organic materials from the water passing through
the contact chamber. However, many toxins have very specific
structures, being toxic specifically because they fit exactly
into or onto some important biomolecule in a living organism,
thereby interfering with its normal activity. Even a small
chemical change will likely reduce the toxicity of even a
very potent natural toxin..
As an application of this principle, ozone has been used
to remove toxins from water,30-33
including natural marine toxins.34
Ozone has been shown, for example, to detoxify botulinum toxin
in freshwater at concentrations of 0.01 ppm ozone and a contact
time of less than a minute.32
Does ozone's reaction with organic toxins impact reef aquaria?
Unfortunately, it isn't possible to answer that. It isn't
even known if such toxins ever become significant in reef
aquarium water. If they do, the answer will depend on
the exact structures of the particular toxin(s) involved.
Ozone may be beneficial from this standpoint, and it is very
unlikely to make such problems worse, but using activated
carbon may be a more effective method than ozone for toxin
removal.
Reducing Bacteria When Using
Ozone
Bacteria and other organisms suspended
in water can be killed by adequate exposure to ozone. That
process is widely used to disinfect drinking water and wastewater
in a variety of applications. The doses and exposures of ozone
required for disinfection, however, are quite high. They are
higher than are used in reef aquarium applications, where
typical doses of ozone range up to about 0.3 ppm in typical
contact chambers, and last for only a few seconds. Consequently,
aquarists must be careful when translating disinfection literature
to reef aquarium effects.
In a recent study of a recirculating seawater system,35
the dosing of 0.52 ppm of ozone was tested for its ability
to decrease the system's bacterial load. That dose is similar
to a 300 mg/hr ozone unit applied to a typical small skimmer
flow rate of 150 gallons per hour (568 L/h). In this experiment,
the levels of suspended bacteria (both Vibrio and coliform)
were analyzed in a variety of locations (intake, pre-ozone,
post-ozone, pre-tank, and post-tank). In no case was there
a statistically significant reduction in bacteria. Even the
addition of a venturi injector to the contact chamber did
not adequately help (although it trended toward fewer bacteria,
the result was not statistically significant). For comparison
purposes, at higher ozone concentrations and contact times
(5.3 ppm ozone for 240 minutes), Vibrio vulnificus
is easily killed, with fewer than one in a hundred million
of the initial bacteria remaining.36
How much ozone, and for how long, is required to kill suspended
organisms in seawater? In one study of a suspended dinoflagellate
algae (Amphidinium sp. isolated from Australia's Great
Barrier Reef), it was found that 5-11 ppm ozone for six hours
of exposure was required to kill 99.99% of the organisms.37
While that kill rate is impressive, that exposure is far higher
than is ever achieved in a reef aquarium application. Lower
doses and shorter contact times had smaller effects. A dose
of 2 ppm and a short contact time (with the time not stated
in the paper) showed a reduction in bacteria of abut 98% (which
is still quite significant, but would not be referred to as
disinfection).
Similar results were found for the spores of the bacterium
Bacillus subtilis.38
In this case, doses of 14 ppm ozone for 24 hours were required
to kill 99.99 percent of the spores. In another study 99.9%
of fecal coliforms, fecal streptococci and total coliforms
were killed with 10 ppm ozone and a contact time of 10 minutes.39
The exposure of Vibrio species and Fusarium solani
(bacteria that are pathogenic to shrimp) to 3 ppm ozone for
five minutes killed 99.9% of the bacteria.40
Water from a seawater swimming pool was effectively sterilized
using 0.5-1.0 ppm ozone in a contact tower.41
The data for the disinfection of freshwater systems are much
more extensive, and so include more data at lower contact
times and concentrations. In one experiment at a Rainbow trout
hatchery, the addition of 1-1.3 ppm of ozone with a contact
time of 35 seconds reduced heterotrophic bacteria in the aquarium
water itself by about 40-90%.42
Does the ozone used in a typical reef aquarium application
reduce bacteria? Maybe, but certainly not to the extent required
for disinfection. Still, a reduction of 50% of the living
bacteria could have significant effects. The above study in
the trout hatchery showed that the use of ozone at several
times the typical reef aquarium rate and for about five to
ten times the typical contact time results in such a drop.
While the data are unavailable, I expect that the bacteria
in the water exiting a normal reef aquarium's ozone application
are not decreased by as much as 50%.
It seem reasonable to conclude from such literature studies
that most bacteria that enter the ozone reaction chamber in
a typical reef aquarium application will not be killed by
ozone or its byproducts. If killing bacteria in the water
column is a goal, then a UV (ultraviolet) sterilizer may be
more useful.
Reducing Other Pathogens When
Using Ozone
There has been extensive analysis
of the amount of ozone needed to kill the human pathogen Cryptosporidia
parvum in freshwater. Most such studies are looking for
significant disinfection, but some data points show the effects
at lower doses and contact times, and some researchers have
developed models that suggest the amount of killing at any
dose/time combination.43
For example, at 22° C approximately 63% of the organisms
would be expected to be killed at 1 ppm ozone with a contact
time of one minute. The contact times and concentrations are
inversely related, so at a contact time of six seconds, the
required dose to kill 63% is on the order of 10 ppm ozone.
At 0.3 ppm ozone and a six second contact time, typical for
the high end of reef ozone applications, less than 5% of the
organisms would be expected to be killed.
Many viruses are much easier to inactivate with ozone than
are other pathogens.44 Enteric
adenovirus, for example, is inactivated to the extent of 99.8%
after exposure to 0.5 ppm for 15 seconds.44
Feline calicivirus is inactivated to the extent of 98.6% after
exposure to 0.06 ppm for 15 seconds.44
Poliovirus type 1 was inactivated to 99% within 30 seconds
of contact time at 0.15 ppm ozone.45
Hepatitis A virus was inactivated to the extent of 99.999%
within one minute at 1 ppm ozone.46
Norwalk virus was inactivated by 99.9% in 10 seconds of contact
at 0.37 ppm ozone.47 Adenovirus
type 2 was inactivated by 99.99% by 0.2 ppm ozone with a contact
time of about one minute.48
The eggs of a pathogenic helminth (Ascaris suum) were
killed to the extent of 90% by exposure to 3.5-4.7 ppm ozone
for one hour. One additional hour of exposure killed the remainder.49
It seems reasonable to conclude from such literature studies
that many viruses that enter the ozone reaction chamber in
a typical reef aquarium application may be killed by ozone
or its byproducts. Larger pathogens, however, are likely much
more resistant to ozone, and are unlikely to be killed. For
such ends, a UV sterilizer may be more useful, but still may
not be completely effective.
Toxicity of Ozone Produced Oxidants
(OPOs)
Two sorts of toxicity studies of ozone
produced oxidants (OPOs, such as bromate, hypobromous acid,
etc.) are relevant to reef aquarists. The first involves the
testing of seawater that has been exposed to ozone, and the
second involves the testing of specific compounds dissolved
in seawater that are known to form when using ozone. Most
of the OPOs are unstable, and so have few or no specific toxicity
studies. Bromate (BrO3-)
is the notable exception, and its toxicity is examined in
the next section.
Much of the study of OPOs stems from applications slightly
different from aquaria, and such studies must be viewed in
that light. Often they relate to aquaculture facilities, where
ozone is used at high doses to sterilize the water. Other
studies are done on the disinfection of wastewater using ozone,
another high dose application. Bear in mind that OPOs in reef
aquarium applications will be at a maximum of about 0.3 ppm
in typical reaction chambers, and will be lower (hopefully,
much lower) once the water passes over activated carbon (assuming
it does) and finally enters the aquarium. The concentration
of OPO is always given in terms of the weight of ozone that
produces that amount of oxidant.
In terms of the toxicity of ozonated seawater itself, one
group concluded that fish were relatively insensitive to OPOs:
"Ozonation of estuarine or marine waters can produce
significant amount of bromate…Toxicity studies showed
that the concentrations of bromate which theoretically could
be formed in an ozonated discharge were not toxic to the
early life stages of striped bass (Morone saxatilis)
and juvenile spot (Leiostomus xanthurus)."50
Larvae are, in general, more sensitive to OPOs than are eggs,51
adults or juveniles.52 Japanese
flounder eggs were found to be impacted by OPOs to the extent
that 50% did not hatch after one minute of exposure to 2.2
ppm OPO. Larvae aged 3-15 days were killed to the extent of
50% in 24 hours at 0.02-0.05 ppm OPO. Larvae aged 44 days
were killed to the extent of 50% in 24 hours at 0.15 ppm OPO.
In this case, the larvae were shown to have damage to their
branchial tissues.53
The eggs and larvae of Japanese whiting (Silago japonica)
also have been tested for toxicity by OPOs. In this case,
half of the eggs and larvae died in about 24 hours when exposed
to 0.18 and 0.23 ppm OPOs, respectively.54
Certain microalgae are also relatively insensitive to OPOs
(perhaps to the disappointment of many aquarists). The growth
of the microalgae Tetraselmis chuii was found to be
unaffected at levels up to 0.7 ppm.55
At 1 ppm, growth was impacted negatively.
Toxicity tests of OPOs on shrimp show them to be less sensitive
than fish. Penaeus chinensis and Paralichthys olivaceus
were found to live up to 48 hours at OPO concentrations of
more than 1 ppm, while Bastard halibut (fish) in the same
study lived only three hours at 1 ppm and 48 hours at 0.13
ppm.56
As for other organisms, the damage to the American oyster
(Crassostrea virginica) by OPOs varied with their age.
Even for adults, fecal matter accumulation was reduced at
OPO levels as low as 0.05 ppm.57
The effect of OPOs on rotifers (Brachionus plicatilis)
has also been determined.58
No effect on survival was seen at less than 0.22 ppm OPO,
but effects became significant above that level. The authors
point out that bacteria and other pathogens can be killed
at that level, so rotifer cultures can be used with that amount
of continuous ozone to reduce bacterial contamination.
Are these levels of OPO toxicity important to reef aquarists?
That is difficult to answer without knowing the levels that
are attained in reef aquaria. In a typical ozone application
in reef aquaria that might produce 0.1-0.3 ppm OPO in a reaction
chamber, the levels are quite significant compared to potential
toxicity to fish larvae and other organisms at as little as
0.02-0.05 ppm. After passing the reactor's effluent over activated
carbon, the OPO concentrations should be much lower, but exactly
how low is unclear and will vary considerably in different
setups.
Toxicity of Bromate
The toxicity of ozone and bromate
at "natural" levels in the ocean has been assessed
and usually found to be minimal.59
Few studies have examined the toxicity
of excess bromate itself to marine organisms.60
One review article concluded:
"Bromate toxicity tests on marine animals indicate
the levels of bromate produced by chlorination or ozonation
of power plant cooling waters are not acutely toxic. The
LC50 ranged from 30 ppm bromate for
Pacific oyster, Crassostrea gigas, larva to several
hundred ppm for fish, shrimp and clams."9
One individual study showed that Pacific oysters (Crassostrea
gigas) had abnormal larval development at bromate levels
of 30-300 ppm.61,62 Fertilized
eggs of the oyster Crassostrea virginica were killed
at 1 ppm.63 The clams Protothaca
staminea (littleneck) and Macoma inquinata (bent-nosed)
were killed by 880 ppm.64
The marine dinoflagellate Glenodinium halli showed
changes in population growth at 16 ppm.65
The marine microalgae Isochrysis galbana showed changes
in population growth at 8 ppm.65
The marine
diatom (Skeletonema costatum) showed changes in
population growth at 0.125 to 16 ppm.65
The marine
diatom Thalassiosira pseudonana showed changes
in population growth at 16 ppm.65
The salmon
Oncorhynchus keta was killed at 500 ppm and the perch
Cymatogaster aggregata at 880 ppm.64
Two
shrimp (Pandalus danae and Neomysis awatschensis)
were killed at 880 and 176 ppm, respectively.64
Are these levels of toxicity important to reef aquarists?
That is difficult to answer without knowing the levels that
are attained in typical reef aquaria. The one study in the
literature of bromate in a seawater aquarium, described above,
showed the accumulation of up to 0.6 ppm bromate, although
that was an application in which ozone was used for disinfection,
so it was used at high doses. That level is high enough, however,
to cause toxicity to certain organisms, but not others. In
a typical reef aquarium ozone application, the bromate in
the aquarium water is likely to be much lower. How much lower
will likely depend on the way it is used, especially the dose
and whether it is passed over activated carbon before entering
the aquarium. It may also depend on the other husbandry practices
used in the aquarium, because some procedures (such as denitrification)
may reduce bromate levels. In any case, the potential toxicity
data for bromate support the practice of using activated carbon
after ozone exposure.
The Effect of Activated Carbon
on Ozone Produced Oxidants
In order to reduce ozone's potential
toxicity, aquarists typically try to reduce the OPOs in the
effluent coming from the ozone reaction chamber. There are
a variety of ways to accomplish that, but by far the most
commonly used is passing the water over activated carbon (GAC).
In a previous article on how reverse osmosis/deionizing water
purification systems work on tap water, Reverse
Osmosis/Deionization Systems to Purify Tap Water for Reef
Aquaria, I showed how hypochlorite reacted with activated
carbon. Bromate and hypobromite are expected to react similarly.
The reactions within the activated carbon that break down
these compounds rely on having enough active surface area
and time for these catalytic reactions to take place. How
effective that is in a high flow application such as a skimmer's
effluent is unclear. It is effective in reverse osmosis/deionization
(RO/DI) applications because the flow is low and the carbon's
surface area is very high.
When bromate and hypobromite interact with the activated
carbon's surface, they are broken down into bromide ion (Br-)
and oxygen as shown below for bromate, where C* stands for
the activated carbon and CO* stands for the activated carbon
with an attached oxygen atom.
BrOH
+ C* à
Br- + CO* + H+
Some of the oxidized activated carbon remains, and some
breaks down to produce oxygen (O2):
2CO*
à
2C*
+ O2
Some of the CO* can also break down to CO2
(carbon dioxide) in a noncatalytic breakdown of the OPO, but
that is typically a small fraction of the total. None of these
products of reactions are of significant concern to reef aquarists.
The big question for each aquarist is how effective is the
GAC that is being used? As is true for many things examined
in this field, the studies often have been done at high OPO
concentrations relating to disinfection, and are usually in
freshwater. In one patent application, a GAC bed was used
to reduce the OPO in the water passing through it from 1.1
ppm to less than 0.2 ppm.66
Another group showed that completely removing the bromate
required a contact time with the activated carbon of more
than 15 minutes.67 In this
test and in many others that have been published, older activated
carbon was less effective than new activated carbon. The reason
is that organics occupy portions of the GAC's surface where
bromate and other OPOs are broken down.
A second group studying bromate in drinking water showed
that GAC could remove 78-96% of bromate.68
They found that contact time and age of the carbon were important
parameters affecting the removal percentage.
Besides activated carbon, there are other potential ways
to remove OPO's. In one patent application, researchers have
shown that the water used in aquaculture applications can
be treated with ozone, and then with reducing agents that
react with and destroy these agents, thereby reducing its
toxicity.69 They recommend
sulfite, bisulfite, metabisulfite or thiosulfate for that
purpose, but it clearly is not simple to accomplish this automatically
in a reef aquarium.
Does GAC or any other of these methods work well enough for
reef aquarists to use ozone without undesirable side effects?
The answer likely depends on the care which is used in the
GAC treatment, and the aquarist's tolerance for OPOs to pass
into the aquarium. The answer is likely not well enough when
using the highest doses typically used by aquarists and the
lowest tolerance for OPOs (that is, the lowest levels likely
to cause ANY undesirable effects). Because it is not easy
for most aquarists to measure low concentrations of OPOs,
the most prudent course of action (aside from not using ozone)
is to pass the ozonated aquarium water over as much GAC as
possible before letting it re-enter the aquarium.
Removal of Bromate by Biological
Means
In addition to the methods described
above for removing bromate and other OPOs before they get
to the aquarium, they can also removed by biological processes
taking place in aquaria. In this situation bromate is apparently
the one that builds up in aquarium water. Many studies have
shown that biological filters (bacteria on surfaces) can break
down bromate in ozonized drinking water.70-72
Bacteria living under denitrifying conditions can also reduce
bromate. As was mentioned earlier in the article, there is
at least one study in the literature of bromate in a seawater
aquarium.42 Here the ozone
was used for disinfection, so its doses were high. Nevertheless,
the bromate levels in the Living
Seas exhibit at Walt Disney World's Epcot Center were
found to have risen to about 0.6 ppm. Upon adding a batch
denitrifying system, the bromate and nitrate concentrations
began to drop.
Several conclusions can be drawn from this information:
1. When using ozone it may be prudent to have some denitrification
taking place in the aquarium, either in live rock, live sand
or in special denitrification systems.
2. Conclusions about ozone's safety or suitability, even
if directed at exactly the same organisms in two different
aquaria, may depend on the nature of the other husbandry practices
in the two aquaria. For example, using ozone without GAC may
be fine for 653 particular organisms living in tank A that
also happens to have a large amount of live rock that can
provide denitrification, but that same amount of ozone dosed
to tank B containing the same 653 organisms without as much
live rock may show more toxicity.
Conclusion
Ozone has many effects when used in
a reef aquarium. The most useful of these is the degradation
of organic materials. Most importantly, and quite coincidently
and fortunately for aquarists, the colored organic pigments
in marine aquaria are very sensitive to ozone. For this reason,
ozone can remove seawater's color quite readily, and much
more effectively than it removes the overall load of organic
material. Its effects on water clarity described by most aquarists
range from minimal to very dramatic, with most aquarists reporting
significant beneficial effects.
Another big effect of ozone is the bioavailability of the
organics in the water. Many organics in the aquarium are not
readily metabolized by bacteria, and such materials may last
for hundreds or thousands
of years in the ocean. Ozone, however, has the ability
to make many organic materials more readily absorbed and metabolized
by bacteria. So in a sense, ozone triggers a bacterial attack
that can reduce the load of circulating organic materials.
This reduction in organic materials may also usefully apply
to circulating toxins released by the aquarium inhabitants
in an effort to kill each other with chemicals.
Ozone and its byproducts can, in high enough doses, kill
many pathogens. The levels of ozone encountered in reef aquaria,
however, may be inadequate to have any significant effect
on total bacterial populations. Viruses are more susceptible
than bacteria to ozone, and they may be effectively inactivated
by typical use. Larger pathogens and parasites are much harder
to kill and are not likely to be killed by ozone in reef aquaria.
Ozone also has a dark side. When reacted with seawater, ozone
produces a variety of highly oxidized halogens such as BrOH
and BrO3-. If the ozone
produced oxidants are not largely removed with activated carbon,
they may enter the aquarium and be hazards to the most sensitive
organisms in the aquarium (which are likely eggs or early
stage larvae).
Finally, ozone alters a variety of other inorganic materials
in ways that may or may not be important. It alters the aquarium's
redox balance, raising the ORP (which may mean as little as
altering the ratios of different forms of manganese in solution).
It may permit more rapid conversion of ferrous ion to ferric
ion, and may increase its bioavailability, but perhaps decrease
the lifetime of strongly complexed iron such as EDTA iron.
Ozone also oxidizes ammonia to nitrate. While that is likely
beneficial, it may alter the relative effectiveness of different
nitrogen export pathways (macroalgae vs. denitrification,
for example). It may drive the speciation of iodine toward
iodate and away from iodide. Is that good or bad? I expect
neither, although others have different opinions, but it is
a good poster child for the many things that happen in reef
aquaria when using ozone that normally take place without
any notice or recognition of them by the aquarist.
So with all things considered, is the use of ozone in a reef
aquarium worthwhile? Many aquarists answer with a resounding,
"Yes!" I'll leave that question unanswered until
additional information is detailed in the next two articles
discussing what equipment and methods are most useful for
applying ozone to aquaria, and reporting on what impact it
had in my aquarium.
Until then,
Happy Reefing!
|
If you have any questions
about this article, please visit my author forum
on Reef Central.
|
|
References:
1. Manganese oxides as a redox buffer of natural waters.
Pokrovskii, O. S. Moskovskii Gosudarstvennyi Universitet,
Moscow, Russia. Geokhimiya (1996), (4), 338-344. or, Manganese
dioxide as a redox buffer in natural waters. Pokrovsky,
O. S. Department of Geography, Moscow State University, Russia.
Editor(s): Kharaka, Yousif K.; Chudaev, Oleg V. Water-Rock
Interaction, Proceedings of the International Symposium on
Water-Rock Interaction, 8th, Vladivostok, Aug. 15-19, 1995
(1995), 749-50. Publisher: Balkema, Rotterdam, Neth.
2. Ozone deposition to the sea surface: chemical enhancement
and wind speed dependence. Chang, Wonil; Heikes, Brian
G.; Lee, Meehye. Department of Earth and Environmental Sciences,
Korea University, Seoul, S. Korea. Atmospheric Environment
(2004), 38(7), 1053-1059. Publisher: Elsevier Science B.V.
3. Ozonation of drinking water: Part II. Disinfection
and by-product formation in presence of bromide, iodide or
chlorine. von Gunten, Urs. EAWAG, Swiss Federal Institute
for Environmental Science and Technology, Dubendorf, Switz.
Water Research (2003), 37(7), 1469-1487. Publisher: Elsevier
Science Ltd.
4. Oxidation of Iodide and Hypoiodous Acid in the Disinfection
of Natural Waters. Bichsel, Yves; von Gunten, Urs. Swiss
Federal Institute for Environmental Science and Technology
EAWAG, Duebendorf, Switz. Environmental Science and Technology
(1999), 33(22), 4040-4045. Publisher: American Chemical Society.
A wide product range and reasonable prices are waiting for you on
Tesco Offers.
5. Evidence for the occasional appearance of molecular
iodine in sea water. Moeller, A.; Lovric, M.; Scholz,
F. Institut fur Angewandte Analytik und Umweltchemie, Humboldt-Universitat
zu Berlin, Berlin, Germany. International Journal of Environmental
Analytical Chemistry (1996), 63(2), 99-106. Publisher: Gordon
& Breach.
6. Ozonation of seawater: preliminary observations on
the oxidation of bromide, chloride and organic carbon.
Williams, P. M.; Baldwin, R. J.; Robertson, K. J. Inst. Mar.
Resour., Univ. California, La Jolla, CA, USA. Water Research
(1978), 12(6), 385-8.
7. Bromate formation during ozonation of bromide-containing
waters. von Gunten, U.; Hoigne, J.; Bruchet, A. Swiss
Federal Institute for Water Resources and Water Pollution
Control, Duebendorf, Switz. Water Supply (1995), 13(1), 45-50.
8. Interaction of ozone with sodium chloride - a possible
additional source of chlorine in the atmosphere. Levanov,
A. V.; Antipenko, E. E.; Zosimov, A. V.; Lunin, V. V. Chemistry
Department, Lomonosov Moscow State University, Russia. NATO
Science Series, IV: Earth and Environmental Sciences (2002),
16(Global Atmospheric Change and Its Impact on Regional Air
Quality), 159-162. Publisher: Kluwer Academic Publishers.
9. Measurements of oxidants in ozonized sea water and
some biological reactions. Crecelius, Eric A. Mar. Res.
Lab., Battelle, Pac. Northwest Lab., Sequim, WA, USA. Journal
of the Fisheries Research Board of Canada (1979), 36(8), 1006-8.
10. Modeling of nitrate and bromate in a seawater aquarium.
Grguric, Gordan; Coston, Christopher J. Marine Science Program,
Richard Stockton College, Pomona, NJ, USA. Water Research
(1998), 32(6), 1759-1768. Publisher: Elsevier Science, Ltd.
11. Effects of ozonation on the speciation of dissolved
iodine in artificial seawater. Sherrill, Johanna; Whitaker,
Brent R; Wong, George T. F. Department of Animal Health, Smithsonian
National Zoological Park, 3001 Connecticut Avenue, Washington,
D.C. 20008, USA. Journal of zoo and wildlife medicine: official
publication of the American Association of Zoo Veterinarians
(2004), 35(3), 347-55.
12. Kinetic studies of removal of ammonia from seawater
by ozonation. Tanaka, Junko; Matsumura, Masatoshi. Institute
of Applied Biochemistry, University of Tsukuba, Tsukuba, Japan.
Journal of Chemical Technology and Biotechnology (2002), 77(6),
649-656. Publisher: John Wiley & Sons, Ltd.
13. Residual oxidant decay and bromate formation in chlorinated
and ozonated seawater. Richardson, Leonard B.; Burton,
Dennis T.; Helz, George R.; Rhoderick, John C. Benedict Estuarine
Res. Lab., Acad. Nat. Sci. Philadelphia, Benedict, MD, USA.
Water Research (1981), 15(9), 1067-74.
14. Oxidation of Fe2+
by ozone in the well-mixed semicontinuous reactor. Schreiber,
Andre. Univ. Natl. Zaire, Lubumbashi, Zaire. Chimia (1972),
26(2), 77-9.
15. Relative stoichiometry of the oxidation of ferrous
ion by ozone in aqueous solution. Yang, T. C.; Neely,
W. C. Dep. Chem., Auburn Univ., Auburn, AL, USA. Analytical
Chemistry (1986), 58(7), 1551-5.
16. Oxidation of ferrous ions by ozone in acidic solutions.
Loegager, Tine; Holcman, Jerzy; Sehested, Knud; Pedersen,
Thorvald. Dep. Environ. Sci. Technol., RISOE Natl. Lab., Roskilde,
Den. Inorganic Chemistry (1992), 31(17), 3523-9.
17. Kinetic Model for Fe(II) Oxidation in Seawater in
the Absence and Presence of Natural Organic Matter. Rose,
Andrew L.; Waite, T. David. School of Civil and Environmental
Engineering, The University of New South Wales, Sydney, Australia.
Environmental Science and Technology (2002), 36(3), 433-444.
Publisher: American Chemical Society.
18. Effects of tertiary methods on total organic carbon
removal in saline, closed-system marine mammal pools.
Adams G; Spotte S. American journal of veterinary research
(1980), 41(9), 1470-4.
19. Rate constants of reactions of ozone with organic
and inorganic compounds in water. I. Non-dissociating
organic compounds. Hoigne, J.; Bader, H. Fed. Inst. Water
Resourc. Water Pollution Control, Swiss Fed. Inst. Technol.,
Dubendorf, Switz. Water Research (1983), 17(2), 173-83.
20. Treatment of humic waters by ozone. Floegstad,
H.; Oedegaard, H. Found. Sci. Ind. Res., Norw. Inst. Technol.,
Trondheim, Norway. Ozone: Science & Engineering (1985),
7(2), 121-36.
21. Laser Tweezers Raman Study of Optically Trapped Aerosol
Droplets of Seawater and Oleic Acid Reacting with Ozone: Implications
for Cloud-Droplet Properties. King, Martin D.; Thompson,
Katherine C.; Ward, Andrew D. Department of Geology, Royal
Holloway University of London, Egham, Surrey, UK. Journal
of the American Chemical Society (2004), 126(51), 16710-16711.
Publisher: American Chemical Society.
22. Laboratory Perspectives on the Chemical Transformations
of Organic Matter in Atmospheric Particles. Rudich, Yinon.
Department of Environmental Sciences, Weizmann Institute,
Rehovot, Israel. Chemical Reviews (Washington, DC, United
States) (2003), 103(12), 5097-5124. Publisher: American Chemical
Society.
23. Mechanism of the reaction of ozone with phenol.
Konstantinova, M. L.; Razumovskii, S. D.; Zaikov, G. E. Inst.
Khim. Fiz. im. Semenova, Moscow, USSR. Izvestiya Akademii
Nauk SSSR, Seriya Khimicheskaya (1991), (2), 324-8.
24. The reaction kinetics, decomposition pathways and
intermediate formations of phenol in ozonation, UV / O3
and UV / H2O2
processes. Huang, Ching-Rong; Shu, Hung-Yee. Department
of Chemical Engineering, Chemistry and Environmental Science,
New Jersey Institute of Technology, Newark, NJ, USA. Journal
of Hazardous Materials (1995), 41(1), 47-64.
25. Rate constants for the reactions of ozone with chlorophenols
in aqueous solutions. Benitez, F. J.; Beltran-Heredia,
J.; Acero, J. L.; Rubio, F. J. Departamento de Ingenieria
Quimica y Energetica, Universidad de Extremadura, Badajoz,
Spain. Journal of Hazardous Materials (2000), 79(3), 271-285.
Publisher: Elsevier Science B.V.
26. Comparison of different advanced oxidation processes
for phenol degradation. Esplugas, Santiago; Gimenez, Jaime;
Contreras, Sandra; Pascual, Esther; Rodriguez, Miguel. Departamento
de Ingenieria Quimica y Metalurgia de la Universidad de Barcelona,
Barcelona, Spain. Water Research (2002), 36(4), 1034-1042.
27. Microbially available organic carbon, phosphorus,
and microbial growth in ozonated drinking water. Lehtola,
M. J.; Miettinen, I. T.; Vartiainen, T.; Myllykangas, T.;
Martikainen, P. J. Laboratory of Environmental Microbiology,
P.O. Box 95, National Public Health Institute, Kuopio, Finland.
Water Research (2001), 35(7), 1635-1640.
28. Investigation of microbially available phosphorus
(MAP) in Flemish drinking water. Polanska, Monika; Huysman,
Koen; Van Keer, Chris. KaHo Sint-Lieven, Laboratory for Microbiology,
Ghent, Belg. Water Research (2005), 39(11), 2267-2272.
29. Effect of ozone pre-treatment of colored upland water
on some biological parameters of sand filters. Yordanov,
R. V.; Melvin, M. A. L.; Law, S. P.; Littlejohn, J.; Lamb,
A. J. School of Applied Sciences, The Robert Gordon University,
Aberdeen, UK. Ozone: Science & Engineering (1999), 21(6),
615-628.
30. Optimisation of reactor technology for selective oxidation
of toxic organic pollutants in wastewater by ozone. Rulkens,
Wim H.; Bruning, Harry; Boncz, Marc A. Sub-department of Environmental
Technology, Wageningen University and Research Centre, Wageningen,
Neth. Environmental Science Research (2005), 59(Chemistry
for the Protection of the Environment), 255-273. Publisher:
Springer Science + Business Media, Inc.
31. Decolourization and detoxification of reactive dye-bath
effluents by ozonation. Arslan-Alaton, Idil; Cokgor, Emine
Ubay; Ongunsu, Ilga; Akakinci, Aybars; Sahin, Atilla. Civil
Engineering Faculty Environmental Engineering Department,
Istanbul Technical University, Istanbul, Turk. Fresenius Environmental
Bulletin (2004), 13(10), 1049-1052.
32. Ozone inactivation of botulinum type E toxin.
Graikoski, John T.; Blogoslawski, Walter J.; Choromanski,
Joseph. Northeast Fish. Cent., Natl. Mar. Fish. Serv., Milford,
CT, USA. Ozone: Science & Engineering (1984), 6(4), 229-34.
33. Study on the removal of toxic substance from river
water using O3-GAC process. Yang,
Yu-nan; Sun, Zhi-rong; Wang, Bao-zhen; Yang, Min; Li, Wen-lan.
Research Center for Eco-Environmental Sciences, Chinese Academy
of Sciences, Beijing, Peop. Rep. China. Journal of Harbin
Institute of Technology (English Edition) (2004), 11(4), 447-451.
34. Selective reactivity, virucidal and detoxifying activity
of ozone. Viebahn, Renate. Iffezheim, Fed. Rep. Ger. Erfahrungsheilkunde
(1979), 28(10), 775-82.
35. Microbial analysis of ozone disinfection in a recirculating
seawater system. Hsieh, Jennifer L; Chikarmane, Hemant
M; Smolowitz, Roxanna; Uhlinger, Kevin R; Mebane, W; Kuzirian,
Alan M. Marine Biological Laboratory, Woods Hole, Massachusetts
02543, USA. Biological bulletin (2002), 203(2), 266-7.
36. Ozone disinfection of Vibrio vulnificus in
artificial seawater. Schneider, K. R.; Steslow, F. S.;
Sierra, F. S.; Rodrick, G. E.; Noss, C. I. Dep. Food Sci.
Hum. Nutr., Univ. Florida, Gainesville, FL, USA. Ozone: Science
& Engineering (1990), 12(4), 423-36.
37. Ozonation of the marine dinoflagellate alga Amphidinium
sp. - implications for ballast water disinfection. Oemcke,
D. J.; van Leeuwen, J. Provisor Pty Ltd., Glen Osmond, Australia.
Water Research (2005), 39(20), 5119-5125. Publisher: Elsevier,
Ltd.
38. Seawater ozonation of Bacillus subtilis spores:
Implications for the use of ozone in ballast water treatment.
Oemcke, D.; van Leeuwen, J. Hartley Grove Urrbrae, Australia.
Ozone: Science & Engineering (2004), 26(4), 389-401. Publisher:
Taylor & Francis, Inc.
39. Comparative evaluation of UV, O3
and PAA for waste water disinfection. Pommepuy, M.; Rudolph,
K.-U. Institut fur Umwelttechnik und Management an der Universitat,
Witten, Germany. European Water Management (1999), 2(3), 44-50.
40. Preliminary results of ozone disinfection of sea water
containing the potential shrimp pathogens Vibrio species
and Fusarium solani. Danald, D. A.; Ure, J.; Lightner,
D. V. Environ. Res. Lab., Tucson Int. Airport, Tucson, AZ,
USA. Ozone: Science & Engineering (1980), 1(4), 329-34.
41. Ozone water treatment at a Scarborough swimming pool.
Overfield, H. V. Water & Water Eng. (1943), 46, 427-32.
42. Ozonation of a recirculating rainbow trout culture
system 1. I. Effects on bacterial gill disease and heterotrophic
bacteria. Bullock, Graham L.; Summerfelt, Steven T.; Noble,
Alicia C.; Weber, Amy L.; Durant, Martin D.; Hankins, Joseph
A. P.O. Box 1746, The Conservation Fund's Freshwater Institute,
Shepherdstown, WV, USA. Aquaculture (1997), 158(1,2), 43-55.
Publisher: Elsevier Science B.V.
43. Development of a Ct equation for the inactivation
of Cryptosporidium oocysts with ozone. Clark, Robert
M.; Sivagenesan, Mano; Rice, Eugene W.; Chen, Jimmy. National
Risk Management Research Laboratory, US Environmental Protection
Agency, Cincinnati, OH, USA. Water Research (2002), 36(12),
3141-3149. Publisher: Elsevier Science Ltd.
44. Inactivation of enteric adenovirus and feline calicivirus
by ozone. Thurston-Enriquez, Jeanette A.; Haas, Charles
N.; Jacangelo, Joseph; Gerba, Charles P. United States Department
of Agriculture-Agricultural Research Service, University of
Nebraska East Campus, Lincoln, NE, USA. Water Research (2005),
39(15), 3650-3656. Publisher: Elsevier B.V.
45. Water disinfection: a review with some consideration
of the requirements of the Third World. Ellis, K. V. Dep.
Civ. Eng., Loughborough Univ. Technol., Leicestershire, UK.
Critical Reviews in Environmental Control (1991), 20(5-6),
341-407.
46. Effects of ozone treatment on the infectivity of hepatitis
A virus. Vaughn, James M.; Chen, Yu Shiaw; Novotny, James
F.; Strout, Deborah. Coll. Med., Univ. New England, Biddeford,
ME, USA. Canadian Journal of Microbiology (1990), 36(8), 557-60.
47. Reduction of Norwalk virus, Poliovirus 1, and bacteriophage
MS2 by ozone disinfection of water. Shin, Gwy-Am; Sobsey,
Mark D. Department of Environmental Sciences and Engineering,
University of North Carolina at Chapel Hill, Chapel Hill,
NC, USA. Applied and Environmental Microbiology (2003), 69(7),
3975-3978.
48. Inactivation of microbial contaminants in the USEPA's
drinking water contaminant candidate list (CCL) by various
disinfectants. Riley, K.R.; Gramos, D. M.; Gerba, C. P.;
Nwachuku, N. Presented at the Proceedings of the International
Water Association's Disinfection 2002 Meeting, St. Petersburg,
FL.
49. Destruction of helminth (Ascaris suum) eggs
by ozone. de Velasquez, Ma. Teresa Orta; Martinez, Jose
L.; Monje-Ramirez, Ignacio; Rojas-Valencia, Ma. Neftali. Instituto
de Ingenieria, Coordinacion de Ingenieria Ambiental, Universidad
Nacional Autonoma de Mexico, Mexico, D. F., Mex. Ozone: Science
& Engineering (2004), 26(4), 359-366.
50. An investigation of chemistry and toxicity of ozone-produced
oxidants and bromate to selected estuarine species. Burton,
Dennis T.; Richardson, Leonard B. Dep. Limnol., Acad. Nat.
Sci., Philadelphia, PA, USA. Avail. NTIS. Report (1981), (EPA-600/4-81-040;
Order No. PB82-116351), 100 pp. From: Gov. Rep. Announce.
Index (U. S.) 1982, 82(3), 571.
51. Toxicity of dissolved ozone to fish eggs and larvae.
Asbury, Clyde; Coler, Robert. Univ. Massachusetts, Amherst,
MA, USA. Journal - Water Pollution Control Federation (1980),
52(7), 1990-6.
52. Ozonation of seawater - applicability of ozone for
recycled hatchery cultivation. Ozawa, T.; Yotsumoto, H.;
Sasaki, T.; Nakayama, S. Cent. Res. Lab., Mitsubishi Electr.
Co., Amagasaki, Japan. Ozone: Science & Engineering (1991),
13(6), 697-710.
53. Acute toxicity of ozone -exposed seawater and chlorinated
seawater for Japanese flounder, Paralichthys olivaceus,
eggs, larvae and juveniles. Mimura, Gen; Katayama, Yasuto;
Ji, Xiangrong; Xie, Jialin; Namba, Kenji. Ebara Jitsugyo Aquaculture
Engineering Lab, Nakahara, Kawasaki, Kanagawa, Japan. Suisan
Zoshoku (1998), 46(4), 569-578. Publisher: Nippon Suisan Zoshoku
Gakkai.
54. Acute toxicity of ozone -produced oxidants to eggs
and larvae of Japanese whiting Sillago japonica.
Isono, Ryosuke S.; Itoh, Yasuo; Kinoshita, Hideaki; Kido,
Katsutoshi. Cent. Lab., Mar. Ecol. Res. Inst., Chiba, Japan.
Nippon Suisan Gakkaishi (1993), 59(9), 1527-33.
55. Influence of ozone-produced oxidant in seawater on
the growth of Tetraselmis chuii. Zheng, Bo; Ma,
Shen; Wang, Chenggang; Gu, Xiangwei. Aquaculture Res. Lab.,
Ocean Univ. Qingdao, Tsingtao, Peop. Rep. China. Qingdao Haiyang
Daxue Xuebao (2002), 32(2), 212-218. Publisher: Qingdao Haiyang
Daxue Xuebao Bianjibu.
56. Toxicity of ozonated sea water to Penaeus chinensis
and Paralichthys olivaceus. Jiang, Guoliang; Liu,
Yun; Yang, Dong; Lu, Yan. College of Marine Life Sciences,
Ocean University of Qingdao, Tsingtao, Peop. Rep. China. Haiyang
Kexue (2001), 25(3), 11-13. Publisher: Kexue Chubanshe.
57. A comparison of ozone and chlorine toxicity to three
life stages of the American oyster Crassostrea virginica.
Richardson, Leonard B.; Burton, Dennis T.; Stavola, Ann M.
Benedict Estuarine Res. Lab., Acad. Nat. Sci. Philadelphia,
Benedict, MD, USA. Marine Environmental Research (1982), 6(2),
99-113.
58. Tolerance of the rotifer Brachionus plicatilis
to ozone and total oxidative residuals. Davis, D. Allen;
Arnold, C. R. Port Aransas, TX, USA. Ozone: Science &
Engineering (1997), 19(5), 457-469. Publisher: Lewis Publishers.
59. Environmental assessment of the impact of ozone to
the neuston of the sea-surface microlayer of the Gulf of Mexico.
Lugo-Fernandez, Alexis; Roscigno, Pasquale F. Minerals Management
Service, Gulf of Mexico OCS Region, Office of Leasing and
Environment, New Orleans, LA, USA. Environmental Monitoring
and Assessment (1999), 55(2), 319-346.
60. Bromate and chlorate - evaluation of potential effects
in aquatic organisms and derivation of environmental quality
standards. Hutchinson, Thomas H.; van Wijk, Dolf J. Brixham
Environmental Laboratory, Devon, UK. Modern Chlor-Alkali Technology
(1998), 7, 26-32.
61. Study No.AB724/B, Brixham Environmental Laboratory, Devon,
London: 3 p.
62. J.Fish.Res.Board Can. 36(8):1006-1008.
63. By-products of oxidative biocides: toxicity to oyster
larvae. Stewart, M. E.; Blogoslawski, W. J.; Hsu, R. Y.;
Helz, G. R. Sch. For. Environ. Stud., Yale Univ., New Haven,
CT, USA. Marine Pollution Bulletin (1979), 10(6), 166-9.
64. J. Fish. Res. Board Can. 36(8):1006-1008.
65. R.L. Jolley, H. Gorchev, and D.H. Hamilton (Eds.), Water
Chlorination: Environmental Impact and Health Effects, Ann
Arbor Sci.Publ., Ann Arbor, MI 2:307-310.
66. Removal of residual oxidants from water. Ota,
Toshiyuki; Matsuo, Hideto; Enomoto, Tomoyuki; Nakagawa, Kunihiko.
(Mitsubishi Heavy Industries, Ltd., Japan). Jpn. Kokai Tokkyo
Koho (1992), 4 pp.
67. Formation and control of bromate. An, Dong; Li,
Wei-guang; Cui, Fu-yi; Song, Jia-xiu; He, Xin. School of Municipal
& Environmental Engineering, Harbin University of Technology,
Harbin, Peop. Rep. China. Shuichuli Jishu (2005), 31(6), 54-55,
78.
68. Removal of bromate and assimilable organic carbon
from drinking water using granular activated carbon. Huang,
W. J.; Peng, H. S.; Peng, M. Y.; Chen, L. Y. Department of
Environmental Engineering, HungKuang University, Sha-Lu, Taichung,
Taiwan. Water Science and Technology (2004), 50(8), 73-80.
69. Chemical reduction in ozonization purification of
seawater for mariculture. Grimberg, Aurelie; Rabillier,
Jean-Marc. (L'Air liquide S. A, Fr.). Eur. Pat. Appl. (2001),
10 pp.
70. Biological reduction of perchlorate and bromate at
low concentrations in an activated carbon filter after the
ozonation process. Liang, Sun; Min, Joon H.; Snoeyink,
Vernon L. Metropolitan Water District of Southern California,
La Verne, CA, USA. Proceedings - Annual Conference, American
Water Works Association (2000), 2139-2156. Publisher: American
Water Works Association.
71. Bromate removal during transition from new granular
activated carbon (GAC) to biological activated carbon (BAC).
Asami, Mari; Aizawa, Takako; Morioka, Takayuki; Nishijima,
Wataru; Tabata, Akihisa; Magara, Yasumoto. Department of Water
Supply Engineering, National Institute of Public Health, Tokyo,
Japan. Water Research (1999), 33(12), 2797-2804. Publisher:
Elsevier Science Ltd.
72. Reduction of bromate by granular activated carbon.
Kirisits, Mary Jo; Snoeyink, Vernon L.; Kruithof, Joop C.
3230 Newmark Civil Engineering Laboratory, University of Illinois
at Champaign-Urbana, Urbana, IL, USA. Editor(s): Wilson, Thomas
E. Water Resources and the Urban Environment--98, Proceedings
of the National Conference on Environmental Engineering, Chicago,
June 7-10, 1998 (1998), 350-355. Publisher: American Society
of Civil Engineers.
|
|
|