"There remains the
undoubted, well demonstrated, fact that corals are most highly
adapted for the capture and extremely rapid digestion of exclusively
animal prey and
that, in relation to the bulk of the tissues, they have a
literally enormous feeding surface,
in most species only exposed at night when zooplankton is
abundant."
Yonge, 1963
Thus far in this somewhat interrupted series
of articles, I have discussed the food of coral reefs, the
role of zooxanthellae and light in supplying corals with nutrition,
and the role of phytoplankton in coral nutrition. In this
article, I will discuss the contributions of zooplankton.
Future articles in this series will treat the contributions
of bacterioplankton, dissolved material, and particulate material
and their roles as a food resource to corals.
As a brief review of my earlier articles,
corals are polytrophic (or mixotrophic) in that they are able
to acquire energy from multiple sources. They are able to
provide themselves with nutrition from the photosynthetic
products (photosynthate) of their zooxanthellae, although
the majority of this material is carbon rich and nitrogen
poor. As such, it has been described as "junk food,"
providing the quick energy needed for their respiration and
much of the excess carbon lost as mucus. Most studies of corals
refer to carbon in terms of animal metabolism or respiration.
However, nitrogen is what is required for protein synthesis,
growth and reproduction. While calcification, or skeletal
growth, is dramatically increased because of the energy provided
by zooxanthellae, other aspects of the coral's needs, such
as tissue growth, ability to successfully compete and repair
injuries, and sexual reproduction is highly dependent on protein
production. As photosynthate does not provide enough nitrogen
to meet their daily needs, feeding on various sources of material,
such as zooplankton, is needed by corals to make up for this
deficit. Additionally, feeding provides the source for any
required trace elements, essential amino acids which are not
able to be synthesized by the coral, vitamins, phosphate,
and other elements. In other words, feeding is absolutely
essential for the survival of corals. The notion that corals
are able to exist without feeding, or by light only, is incorrect
in all cases, with different species relying to varying degrees
on energy acquisition from multiple sources (light, zooplankton,
etc.). The relative amounts of energy provided by a given
source will depend on the relative levels of those things
at any one time, the specific qualities of the species (some
naturally depend more on one thing than another), and various
other environmental and behavioral influences and adaptations.
Introduction
Zooplankton are defined as small (often
microscopic) aquatic animals and nonphotosynthetic protists
suspended or weakly swimming in water. Zooplankton form an
important food (trophic) resource to many groups of animals,
are part of a complex food web, and are themselves significant
consumers of phytoplankton, bacterioplankton and other zooplankton.
There are numerous ways to describe zooplankton; they can
be grouped according to their size, taxonomy, habitat, and
other characteristics (Figure 1).
|
Zooplankton
Characterizations
|
| Holoplankton
|
Plankton
that remains free-swimming through all stages of
its life cycle |
| Meroplankton
|
Any
of various organisms that spend part of their life
cycle, usually the larval or egg stages, as plankton. |
| Demersal
(epibenthic) |
Dwelling
at or near the bottom of a body of water. |
| Pelagic
|
Living
in the water column of open oceans or seas. |
| Mesoplankton
|
Plankton
that grows in deep sea locations. |
| Macroplankton |
Plankton
larger than 1mm (e.g. fish larvae, most copepods,
most mysids, krill, larvacean tunicates, ctenophores,
medusae, gastropod veligers, echinoderm plutei,
chaetognaths, etc). |
| Microplankton
|
plankton
between 1 micrometer and 1 mm in size (1x10-3
m) (e. g. most small invertebrate larvae,
protests). |
| Nanoplankton
|
plankton
between 1 nanometer and 1 micron in size (1x10-6
m). |
| Picoplankton
|
plankton
between 1 picometer and 1 nanometer in size (1x10-9
m).
|
|
Figure
1. Some common classifications of plankton.
|
|
The Occurrence and Types of Zooplankton on Coral
Reefs
Zooplankton, tending to be quite numerous,
albeit small, also accumulate where there is sufficient food
to allow for their growth and reproduction. It is correct
to assume that areas of higher nutrients tend to foster higher
populations of zooplankton, as well. Although certain types
of zooplankton are found in sometimes quite specific areas,
the relative contributions of each group are quite similar.
This is especially true given the overwhelming contributions
of some groups to the plankton (Table 1). Copepods comprise
by far the largest fraction of total zooplankton - more than
all the other groups combined. On coral reefs, demersal copepods
can form dense swarms more than 5 cubic meters in size and
with between 500,000 and 1,500,000 copepods per cubic meter
(Hamner and Carleton 1979). Even with such great densities,
and with demersal zooplankton comprising such a large portion
of the total zooplankton availability on reefs, the amount
of pelagic zooplankton is still astonishingly large. Hamner
et al. (1988) measured zooplankton in the water column flowing
over the reef crest by using a one meter wide trap. During
one day, 0.5kg of plankton was collected across that one meter
strip. Given that most tanks are about one meter wide, the
pelagic fraction of zooplankton alone (not counting the much
greater contribution of demersal zooplankton) would mean an
aquarist would have to dump over a pound of food in their
tank each day to simulate just the small fraction of plankton
available to a reef crest community.
|
Taxonomic
group
|
Number/m-3
|
|
lagoon |
ocean |
| Holoplankton: |
|
|
| copepods |
552.0 |
17.9 |
|
chaetognaths |
23.0 |
0.7 |
|
nauplii, amphipods |
7.0 |
2.0 |
|
appendicularians |
4.8 |
0.1 |
|
ostracods |
0.3 |
23.0 |
|
ctenophore, medusae |
2.1 |
0.05 |
| euphasiids,
amphipods |
0.1 |
0.7 |
|
siphonophores |
0.0 |
0.7 |
| Meroplankton: |
|
|
| annelid
larvae |
0.36 |
0.26 |
|
crab zoea |
0.7 |
0.1 |
| mollusk
veligers |
1.7 |
0.2 |
Table
1. Composition and amount of zooplankton by reef area.
|
It is probably reasonably well known that
coral reef zooplankton are more abundant at night. Much of
the zooplankton on reefs is not pelagic, and is not washed
into the reef environment from the open ocean where levels
are comparatively lower. The majority of reef zooplankton
is demersal, and rises into the water column from the benthos
at night when levels of predation are lower. But, perhaps
the degree to which variations in the relative amounts of
zooplankton occur at night by comparison with levels during
the day might be surprising (Table 2). While some corals feed
during the day, and some feed day and night, the majority
feed at night. This corresponds to when zooplankton is most
abundant. In this way, corals can gain energy from light during
the daytime and feed at night when zooplankton is most abundant.
This is the most energy efficient way for corals to maximize
their energy intake. Of course, exceptions arise over time,
and even night feeding corals may feed during the day, although
it is likely they will only do this if there is sufficient
prey to warrant the considerable energy expense of prey capture.
In aquariums, since there is such a relative paucity of zooplankton
and a concurrent lack of nightly migration, coupled with the
normal daytime feeding of the tank, many normally night-feeding
corals extend to feed during the day. Tentacle extension,
it should be noted, is not always related to food capture,
and may also be indicative of competition or to expose corals
with zooxanthellae in the tentacles to light. Generally, corals
with transparent tentacles (tentacles lacking zooxanthellae),
are night feeders.
| Taxonomic
group |
day
(mg/m-3)
|
night
(mg/m-3)
|
copepods
|
174
|
1574
|
| appendicularians |
4
|
34
|
| chaetognaths
|
2
|
70
|
| amphipods
|
0
|
26
|
| ostracods
|
2.5
|
138
|
| decapods
|
0.7
|
43
|
| veligers |
15
|
382
|
| foraminferans
|
4
|
10
|
| fish
larvae |
13
|
70
|
| mysids
|
6
|
701
|
| crab
zoe |
0
|
237
|
| polychaetes
|
4
|
38
|
|
| Total
Haloplankton/Meroplankton |
130
|
2346
|
Total
Microplankton
(zooflagellates, ciliates, nauplii) |
11
|
181
|
Table
2. Composition and amounts of zooplankton by time of
day.
|
Because it is not known what the energy
budgets of corals in aquariums are, it is difficult to say
whether or not there is an advantage or disadvantage to the
often abnormal feeding behaviors in aquariums. If corals meet
their energy needs through various combinations available
at various times of the day, there is probably little disadvantage.
Nor am I comfortable suggesting the feeding at night is "better"
than feeding during the day. However, it is more natural,
and it may be stressful for those corals that feed almost
exclusively at night in the wild to feed during the day. The
possible deleterious effects of strong lighting on normally
withdrawn tentacles may also be injurious since they probably
lack the photoprotective pigments of the rest of the tissue
and the zooxanthellae.
The Contribution of Zooplankton to Coral Nutrition
In 1995, Sorokin wrote, "The polyps
of scleractinian corals have the largest ratio of
catching area of the body to its biomass among all other aquatic
animals." The debate over the contribution of zooplankton
to coral energy is far from new. C.M. Yonge was the first
to demonstrate that zooxanthellate corals (many diverse species)
could survive "indefinitely" if provided with adequate
zooplankton, even if totally deprived of light. In contrast,
corals provided light and deprived of zooplankton did not
survive. However, in light of increasing study of the zooxanthellae,
it became known that many shallow-water corals could meet
above 100% of their carbon requirements from light alone.
Depending on the study, results indicated often conflicting
data on the relative contributions of light and zooplankton
to coral energy budgets. Some suggested that the zooplankton
contribution was negligible, others suggested it was extensive,
while still others suggested it depending on factors such
as the depth (light availability) or polyp size (large polyped
corals relied more on feeding that small polyped corals).
It is now fairly well established that different species gain
different amounts of energy from the various sources, depending
on many factors that include species-specific differences,
habitat and environment, and the dynamic changes in the availability
of the various resources. However, it is all but conclusively
demonstrated that feeding is required for survival in amounts
that vary from slight to total dependence.
One of the greatest myths among reefkeepers
is that "SPS" corals depend mostly on light, and
require less food than "LPS" corals. This is entirely
untrue. As an example, consider the data from Sebens (1997)
below (Figure 2). This graph shows the capture rate of an
equivalent biomass of two corals, the large-polyped Montastraea
cavernosa and the very small-polyped Madracis mirabilis.
For those unfamiliar with Madracis, it is related to
and somewhat resembles Pocillopora and Stylophora.
The capture rate of the small polyped coral was 36 times greater
than the large-polyped coral! Furthermore, M. cavernosa
has been shown in other studies to be a voracious zooplanktivore.
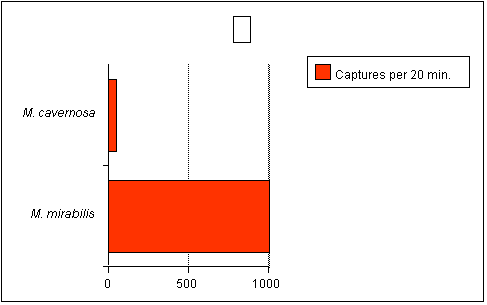 |
|
Figure
2.
|
Number
of zooplankton captured by equivalent biomass of coral
(100 polyps of M. cavernosa, 9000 polyps of M.
mirabilis). Adapted from Sebens (1997).
|
Many other studies confirm the predatory
abilities and requirements of "SPS" corals. It should
not be surprising given the fast growth rate and fecundity
of many small polyped species. In other words, more growth
and reproduction requires more energy, especially nitrogen
for tissue growth. The difference, if one exists between "SPS"
and "LPS" corals, lies primarily in the size of
the food captured. Most of the prey of small polyped corals
may just be too small to see. Aquarists have a tendency to
be strongly visual, and so if gross observations don't indicate
that a coral is consuming food offered to it, they wrongfully
assume the coral must not need to be fed. To further illustrate
the roles of zooplankton capture by stony corals, consider
the data below (Table 3).
| Taxonomic
Group |
Average
calories respired
|
Average
calories ingested
|
| *Manacina
areolata (n=13) |
51.6
|
157.6
|
| *Montastraea
cavernosa (n=11) |
47.1
|
146.8
|
| Porites
porites (n=11) |
59.8
|
247.0
|
| *
indicates large polyped coral |
|
|
Table
3. Average daily respiration and ingestion rates of
Artemia nauplii for three corals. Adopted from
Coles (1997).
|
Three corals were used in this study: Manacina
areolata which is a very large-polyped coral that resembles
the open brain coral, Trachphyllia geoffroyi; the large-polyped
Montastraea cavernosa; and the very small-polyped Porites
porites. The first column represents an approximation
of the metabolic rate of the three species. Porites
respires the most calories, and thus has the highest metabolism.
As might be expected, it also has the highest caloric ingestion
rate. It is notable that all three corals take in roughly
three times what their basic metabolic rate requires. The
additional calories can be used for injury repair, competition,
growth and reproduction, and excess is generally lost as waste
material and mucus. Lest any readers suggest that these are
Caribbean species, and that the situation might be different
in the Pacific, I offer the following data set (Table 4).
| Taxonomic
group |
Daily
respiration
|
Feeding
|
|
(µgC/g
wet)tissue
|
(%
of respiration expenditure)
|
|
|
P
|
DOM
|
B
|
Z
|
Total
|
H/A
|
%
Z
|
| Seriatopora
hystrix |
175
|
140
|
29
|
22
|
250
|
441
|
2.15
|
0.83
|
| Porites
annae |
82
|
80
|
40
|
31
|
100
|
251
|
2.13
|
0.58
|
| Acropora
squamosa |
154
|
140
|
52
|
22
|
200
|
414
|
1.96
|
0.73
|
| Pocillopora
damicornis |
160
|
140
|
28
|
20
|
270
|
458
|
2.27
|
0.85
|
| Hydnophora
exaesa |
100
|
100
|
47
|
75
|
150
|
372
|
2.72
|
0.55
|
| Tubipora
musica |
200
|
70
|
35
|
75
|
70
|
250
|
2.57
|
0.39
|
| Merulina
ampliata |
130
|
90
|
17
|
10
|
160
|
367
|
2.08
|
.086
|
| Leptastrea
transeversa |
90
|
140
|
30
|
85
|
55
|
310
|
1.21
|
0.32
|
| Favites
abdita |
50
|
140
|
17
|
5
|
230
|
392
|
1.80
|
0.91
|
| Galaxea
fascicularis |
90
|
140
|
47
|
20
|
60
|
267
|
0.91
|
0.47
|
| Symphyllia
sp. |
125
|
100
|
22
|
15
|
55
|
192
|
0.92
|
0.60
|
| Fungia
scutaria |
110
|
130
|
24
|
20
|
180
|
354
|
1.72
|
0.80
|
|
|
|
|
|
|
|
|
|
|
P
= Photosynthesis; DOM = Dissolved Organic
Material; B = Bacteria; Z =Zooplankton
(Artemia);
|
|
Total
= average daily energy intake of carbon by photosynthesis
and feeding;
|
|
H/A
= ratio of heterotrophy to autotrophy (higher
number means more heterotrophy);
|
|
%
Z = percentage of feeding on zooplankton to
all heterotrophic sources.
|
Table
4. Respiration and feeding rates of some common corals.
Corals are listed in order
of increasing polyp size.
|
From the original data, I have totaled
the energy inputs to provide a comparison with the Caribbean
species in Table 3. This study is notable in that it considers
only carbon, and not nitrogen. Carbon is typically thought
to be mostly provided by the zooxanthellae photosynthesis.
Despite the fact that most of the corals listed are able to
theoretically meet 100% or more of their daily carbon needs
by light, the zooplankton column shows that an even larger
percentage can be met by feeding on this resource alone -
for all but three of the species, none of which are "SPS"
corals. Notice also how similar most of the species are to
those in Table 3 in that they take in much more than they
require for their daily metabolic needs. I have also given
a ratio of heterotrophy (feeding) to autotrophy (photosynthesis).
The higher the number, the more the coral depends on nutrient
acquisition from feeding by comparison to energy provided
by photosynthesis. Note how there are no noticeable patterns,
but rather the ratios seem to vary simply according to species
and not polyp-size, habitat, or other obvious variable. In
all cases except for Galaxea and Symphyllia,
more energy is acquired by feeding than by photosynthesis,
and for the typical "SPS" species, the ratios tend
to be higher. Finally, I have provided a column that shows
the percentage of feeding on zooplankton compared to feeding
on bacteria or dissolved organic material. Once again, there
are no obvious trends except that some species rely more on
zooplankton than others and that, if anything, the "SPS"
corals feed on zooplankton a lot. In fact, most corals show
linear feeding saturation dynamics under all but extremely
high particle concentrations. What this means is that corals
have a hard time "getting full." They continue to
capture prey and do not get satiated until prey densities
become so great that such levels are almost never possible.
To put it another way, even if you were to pour a pound of
food per day into an average sized reef aquarium, the corals
would still "be hungry." I have so many papers on
zooplankton abundances, composition, and the role of feeding
in corals that to list them all and discuss them would require
an entire book. I have chosen the studies above because they
are typical, useful, and exemplify what could be shown again
and again were I to list and describe other works.
I will finish this very brief coverage
of zooplankton with several recent findings. In a September
2002 coral reef conference in Cambridge, several papers were
presented that should give an idea of not only the very latest
information, but also emphasize what is written above. Many
years ago, one of the only complete energy budgets for a coral
was done for what might be considered the ultimate shallow-water
"SPS" coral, Acropora palmata (Bythell 1988,
1990). The study showed, basically, that 70% of this coral's
nitrogen needs were met by feeding and that 91% of its carbon
needs were met by light. At the 2002 conference, Bythell et
al. examined three more corals, the larger polyped Montastraea
cavernosa, M. annularis and Menadrina meandrites.
They found zooplankton to provide 20-80 times the carbon and
112-460 times the nitrogen previously shown for Acropora
palmata. Finally, Fanny et al. (2002) investigated the
role of zooplankton consumption on the metabolism of the small-polyped
coral, Stylophora pistillata under 3 different conditions
of light (80, 200, 300 µmoles m-2 s-1) and 2 feeding
regimes (Artemia and natural plankton). They found
that regardless of light, fed corals had higher chlorophyll
a concentrations, higher protein levels, and had photosynthesis
rates 2-10 times higher than those deprived of food. This
group also measured calcification rates, both in the dark
and in light, and found that calcification, as is well known
to be the case, is enhanced by light. However, for the first
time it was shown that feeding results in calcification rates
50-75% higher than in control corals (not fed). It was also
found that feeding does not affect the light-enhancement process
of photosynthesis on calcification. To make these results
completely understandable, if corals can feed on zooplankton,
they will calcify 50-75% faster irrespective of light levels
provided.
Conclusion
It has always surprised me the lengths
aquarists go to devise various simulations of natural reef
processes. The amount of ingenuity, effort, and expense spent
on various aquarium devices and products is almost beyond
belief. Aquarists hinge their belief that some bottle of trace
elements or some new color temperature light bulb will increase
the health and growth of their corals, despite scanty or non-existent
evidence of it being true. Of all the many things that can
potentially increase respiration, photosynthesis, and calcification
- and have been show again and again to do so absolutely-
feeding and water flow are the major players. Light, of course,
is critically important as well, but aquarists by and large
can and do provide enough quantity and quality of light for
corals. Period. Phytoplankton, while a very beneficial addition
to aquaria, does not feed most corals (Borneman 2002). Something
as significant as zooplankton to both coral and coral reefs
would seem worthy of the highest efforts in trying to produce,
add, grow, substitute or in some way provide to tanks. I cannot
think of a single greater accomplishment and advance for aquarists
than to provide by whatever means (higher export and higher
input, larger refugia, purchase, plankton tow, culture, etc.)
significantly greater levels of zooplankton or zooplankton
substitutes to their corals. I hope I am being dramatic enough
by writing this, for this is among the most important steps
that must be made to realize the majority of those lofty goals
and ideals that are so often stated and desired by those keeping
corals in aquariums. Similarly, I very much hope that the
information in this article, and provided in additional works
in the bibliography below, gives the slightest inkling of
the predatory capabilities and importance of feeding in all
corals.
"The quality and
fates of coral primary production imply that zooxanthellae
provide "junk food" to their hosts, and beg the
question of nutrient limitation of coral growth rates under
conditions of adequate light…On present evidence it
seems clear that all corals need to supplement their diet
(with food) from outside the symbiosis (heterotrophy) in
order to meet these requirements."
Hatcher, 1988
Links to
Part
1, Part
2, Part
3, Part
5, Part
6, Part
7
|

