|
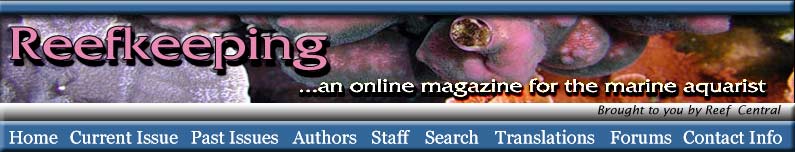
Last month, I discussed the topic of food
sources to coral reefs, and this month I will narrow the subject
down to food sources for corals. Coral feeding is part of
a well-orchestrated “three-part harmony,” because corals are
supremely adapted to utilizing all manner of the available
food sources on coral reefs. The three parts to this story,
or harmony, are light, prey capture, and direct absorption.
This month, I will cover only the first part, the nutritive
aspects of light.
The Energy of Light
Before becoming concerned about a repetition
of a bevy of other articles on the subject by many authors,
this will not be a discussion of aspects of lighting, qualities
of light, suggestions for lighting, or anything of that nature.
Perhaps such subjects are interesting; they certainly have
been well discussed, and presumably because of the vital importance
of light to many corals. Rather, it is my intention here to
have the readers understand exactly why lighting is an important
subject in reef aquaria.
As I mentioned in the last article, there
are two basic types of organisms: autotrophs (mostly photosynthetic
organisms) and heterotrophs. Corals are heterotrophs, with
a big caveat. Most reef building corals, or hermatypes, and
many non-reef building corals, or ahermatypes, maintain symbioses
with various dinoflagellate algae called zooxanthellae. While
the coral polyp itself is not autotrophic, its nearly obligate
association with these dinoflagellates provides polyps with
a built-in autotroph that it can, to some degree, control.
Therefore, reef corals with polyps maintaining symbionts have
characteristics of both autotrophs and heterotrophs. Lighting
provides the energy for zooxanthellae to photosynthesize.
It may or may not come as some surprise that light, to corals,
is simply food.
Also mentioned in the last article was
the fact that the waters around coral reefs usually have extremely
low levels of various nutrient sources, largely because of
fierce competition for those same nutrients amongst the vast
numbers of species found there. A common and successful strategy
to allow successful competition for habitat space in such
an environment is to utilize an energy resource that is not
generally limited in tropical waters... sunlight. Corals are
not the only organisms to utilize this strategy, as clams,
sponges, hydroids, foraminiferans, nudibranchs, and many other
organisms also host photosynthetic algal or bacterial cells
in their tissues for a similar purpose. As it turns out, sunlight
is such a valuable commodity that means to attain as much
of it as possible are built into the life history strategies
and behaviors of organisms harboring such symbionts. For corals,
regulation of the zooxanthellae population is possible, they
expand or contract their tissues to expose more or less zooxanthellae
to sunlight, and they modify their growth forms to those ideally
suited to their “place in the sun.” Accessory animal pigments
are also produced to further modify the light environment
to which corals are exposed.
Zooxanthellate and Azooxanthellate Corals
Corals far outside tropical areas, or those
in very deep water, do not contain zooxanthellae. Oddly enough,
perhaps, is that there are a great many azooxanthellate corals
existing on coral reefs alongside or nearby their brethren
with symbionts. If harboring zooxanthellae is such a successful
strategy, why don’t all corals have these symbionts? Part
of the answer lies in evolution. Perhaps it has not been advantageous
for some species to adopt them, or perhaps not all species
have recently invaded the shallow water zones and have not
had enough evolutionary time to do so.
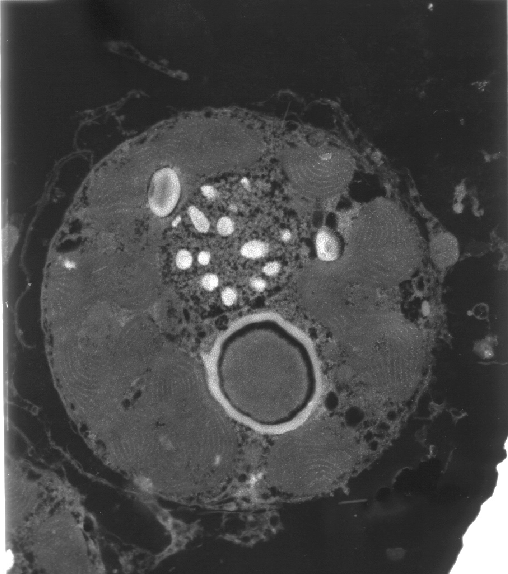 |
A transmission electron micrograph
of a zooxanthella. The areas with parallel bands are
stacked thylakoid membranes of the chloroplasts where
light-harvesting takes place.
|
In fact, there are corals that are facultatively
zooxanthellate; these corals, some from the tropics and some
from sub-tropical regions can exist either with, or without,
zooxanthellae. Commonly researched corals of this type include
some species of Madracis,
Astrangia, and Oculina. In fact, one of the nemeses of aquarists, Aiptasia
pallida, the glass anemone, is also facultatively zooxanthellate.
As it happens, these corals tend to exist with zooxanthellae
in warm, clear, shallow waters and without them in turbid,
cold, or deep waters.
What this means, among other things, is
that if it doesn’t provide much of an advantage to host zooxanthellae
in certain areas, why have them present at all? It could be
argued that some photosynthesis is better than none at all,
even in deep or temperate water. But, perhaps this is not
the case… if there is a cost involved. And, indeed there is
a cost to the organism to maintain zooxanthellae within their
cells. Apparently, there is no such thing as a “free lunch”
for corals, either. These facultative hosts must make a metabolic
choice as to whether the benefits of hosting zooxanthellae
outweigh the costs. In most coral reef environments, the symbiosis
is not so optional, and is usually considered to be nearly
an obligate association. I say nearly, because bleaching is
a prime example of when the costs of maintaining the symbiosis
outweigh the benefits, although the bleaching response is
complex and such a statement represents something of a simplification.
This somewhat answers the question of why
some corals maintain zooxanthellae: they retain benefits of
photosynthesis where prevailing conditions are advantageous
over the costs of maintaining them, such as on coral reefs.
This group includes most of the hermatypic stony corals, most
of the shallow water Caribbean gorgonians, a couple of shallow
water Pacific gorgonians, more than half of the soft corals,
about half of the number of species of zoanthids (although
the vast majority in terms of numbers of organisms), most
of the corallimorphs, and a single genus of hydrocoral (although
this single genus, Millepora,
contains the majority in terms of numbers of organisms on
coral reefs).
It may seem logical that zooxanthellate
species must exist in shallow water to take advantage of sunlight,
but we are still left with one pressing question. How do some
azooxanthellate species seem to compete so well amongst zooxanthellate
species, in particular, some Pacific soft corals like Dendronephthya species and Tubastraea
micranthus? The answer lies in the fact that most probably
compete well with their rapid growth, prolific asexual reproduction,
and other behaviors. For the most part, zooxanthellate corals
do indeed compete their azooxanthellate kin for sunlight-drenched
areas, often resigning those without algal partners to recesses,
caves, nooks, crannies, and seemingly less desirable real
estate. Lest it be thought that azooxanthellate corals are
“inferior,” it is more accurately stated that, like many specialists
that exist on reefs because of their specialization, they
compete well where others cannot. In other words, every organism
finds its place where it tends to be successful. As testimony
to the fact that zooxanthellae are not necessarily the penultimate
adaptation to shallow coral reef waters, at one time in history,
all corals were azooxanthellate and did not compete for the
space to catch sunlight at all.
Types of Zooxanthellae
So successful is the symbiosis between
corals and their zooxanthellae that multiple relationships
have developed. At one time, and not long ago at all, coral
researchers were convinced that all corals held but one type
of symbiont within them. These single celled algae were called,
despite numerous synonymous names, Symbiodinium
microadriaticum. Eventually, several other dinoflagellate
zooxanthellae were found in the fire coral, Millepora, and in some zoanthids. However,
it was still largely assumed that all other corals harbored
a single species of algae. About twenty years ago, the walls
around such a notion began to crumble, and it is now recognized
that there are many clades (groups of biological taxa that
includes all descendants of a common ancestor), such as species,
types, and subtypes of zooxanthellae that inhabit coral tissue.
In fact, so widespread is the diversity beginning to appear
that a complete rewriting of coral symbiosis is beginning,
with only a few introductory chapters written as of today.
The diversity and nature of the various relationships is now
hardly known. What is known is that not only may there be
a variety of one coral/one symbiont relationships, but that
various corals may harbor more than one symbiont, may potentially
be able to harbor more than one symbiont even if it is not
usually found to do so, and that even single corals may harbor
more than one symbiont at the same time. I would refer the
reader to more information in my article here,
even though much of that information has already changed,
so rapid are the advances in this field. There is a very large
body of science regarding this subject, and to cover it in
much more depth without many additional pages, I am afraid,
would be doing the subject an injustice.
What The Symbiosis Provides And How Much
Given the general background above, I can
now delve into the crux of this relationship and describe
just what it means to house autotrophs in a heterotrophic
body. Zooxanthellae are initially acquired either from the
water column (in broadcast spawning corals), or are given
a starter culture from the parent polyp (in brooding corals).
Over the course of their lives, coral polyps maintain various
densities of zooxanthellae in their tissues according to environmental
and metabolic conditions. Polyps periodically release or lose
some, require more from the water column, and control their
growth and reproduction within their tissues quite effectively.
For a description of when the symbiosis does not go quite
as smoothly, a process known as coral bleaching, see this
article.
The algae are maintained mostly in the underlying tissue layer,
the gastrodermis, and within the tentacles of some species,
in small containment vesicles called vacuoles. These vacuoles
are formed within the gut cavity of corals after the dinoflagellates
have been swallowed, and they can even migrate across tissue
layers.
Once in place, the zooxanthellae
reproduce until they form a mostly single layer within the
tissue; an arrangement that maximizes light capture as a photosynthetic
umbrella, or antenna, while minimizing shading of adjacent
algal cells.
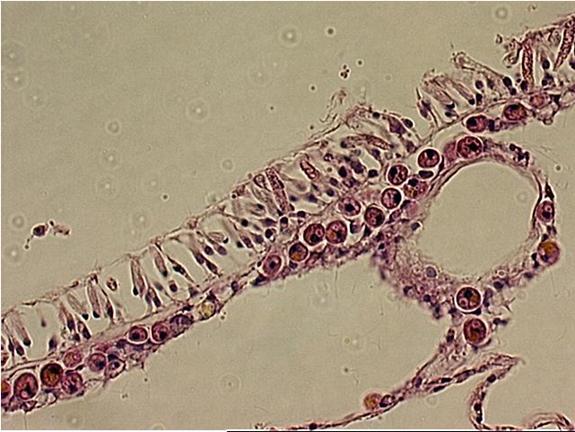 |
The upper layer of the Acropora
sp. is the epidermis. The lower layer is the gastrodermis.
Within the cells are round to oval golden spheres.
These are the zooxanthellae.
|
The zooxanthellae are then carefully controlled
by their coral host by being subjected to nitrogen limitation.
As mentioned in last month’s article, nitrogen levels in coral
reef waters are typically extraordinarily low, with most being
found as ammonia. This is in contrast to aquaria where the
dominant nitrogen species is usually nitrate. Nitrogen is
the end all-be all for zooxanthellae growth and reproduction.
By limiting nitrogen in the form of excretion products, the
polyp keeps the zooxanthellae in the numbers and density that
maximize photosynthetic efficiency for its own use. Using
several released compounds, most of which are still unidentified,
the polyp stimulates the zooxanthellae to release virtually
all of the products of its photosynthesis, and these are then
used by the polyp for its own needs. If nitrogen was made
readily available to the zooxanthellae (for example, if high
levels were present in the water and the dissolved nitrogen
“diffused” into the coral tissue), it could then be accessed
by the algae without limitation by the polyp, and zooxanthellae
could begin to grow and reproduce like a “phytoplankton culture.”
In this case, the symbiosis becomes less advantageous to the
coral, and it will expel some of the symbionts to try and
re-establish maximal benefit from its algal partners. As a
practical note, when very high densities of zooxanthellae
exist in coral tissue, the resultant coloration of the coral
is usually a rich or dark brown color.
This relationship may not sound altogether
“symbiotic.” It may even sound parasitic, since the coral
is clearly taking advantage of the zooxanthellae, and seemingly
without much “giving.” Yet, nitrogen is so limiting on coral
reefs that even the limited excretion of the coral provides
a relatively stable supply, as well as a protected stable
environment, to the zooxanthellae.
Given that corals are “squeezing” their
symbionts for all they are worth, what exactly are they worth?
As it turns out, the symbionts provide a constant “sugar fix.”
The high carbon products of photosynthesis are mostly sugars,
and the coral squeezes out almost 100% of the algal production,
allowing just enough to maintain the algae’s carbon needs
for its survival. In shallow clear water, efficient corals
can get over 100% of their daily carbon needs from their zooxanthellae.
These photosynthetically-derived sugars are then used by the
coral for metabolic functions that require energy, and much
of them are lost in the copious production of mucus. Coral
mucus, in turn, and as was shown in the previous article,
is itself a food source to the reef. The production of mucus
by corals is also very important for their protection, food
acquisition, competition, and other functions.
Unfortunately, zooxanthellae don’t make
much else besides sugar. The coral squeezes out what it can,
but not much more ever results. In particular, nitrogen, once
again, is a problem. It seems everyone on the reef is always
scrambling for nitrogen, the substance needed to produce protein;
proteins required for nematocysts, vitamins, tissue maintenance,
injury repair, cell division, growth, gamete production, even
the very toxins used to paralyze prey. Proteins are the ticket
to growth and reproduction in zooxanthellae, as well as for
coral polyps. Thus, it may come as little surprise that this
great sugar fix provided by symbiotic algae comes up rather
nutritionally short in the course of coral nutrition. To survive
and, hopefully, thrive, corals need more than light. They
need to swallow more than their symbiotic zooxanthellae. And
this will be the subject of next month’s article.
Links to
Part
1, Part
3, Part
4, Part
5, Part
6, Part
7
|