|
Introduction
The past few years in the reef aquarium
hobby has witnessed an explosion in the use and availability
of phytoplankton products. As is my normal mode of operation,
I will not be covering the various advantages or differences
in the various phytoplankton products available. However,
what I will cover are some of the immensely complex and largely
unknown aspects of what phytoplankton are: and what they are
not. I have witnessed the widespread belief that phytoplankton
are unicellular plants that are consumed by corals, and that
the addition of phytoplankton comprises a newly discovered
beneficial supplement for reef aquariums. Unfortunately, that
belief is largely erroneous and highly simplified. I am, by
no means, an expert on phytoplankton - either by science,
or by experience with their uses in aquaria. I would urge
anyone seeking a greater depth of information to seek out
review articles and texts on the subject, of which there are
thousands, or to contact someone with more expertise in the
field. Yet, perhaps something of value may be gained from
the words that follow.
What Are Phytoplankton?
Suspended drifting material in the water
is either living or dead. In the first case, it is commonly
referred to as plankton, and in the latter, detritus. Detritus
will be covered in a future article. The word phytoplankton
comes from the Greek words, phyton, meaning plant, and planktos,
meaning to drift or wander, and combined to mean, "drifting
plant." A problem arises from the fact that the original
name certainly stemmed from the notion that green things that
used sunlight and had cell walls were plants. Unfortunately,
this is not the case. Most of the phytoplankton are in the
Kingdom Protista, or protists, and may share unique attributes
that are neither animal nor plant, or perhaps a bit of both.
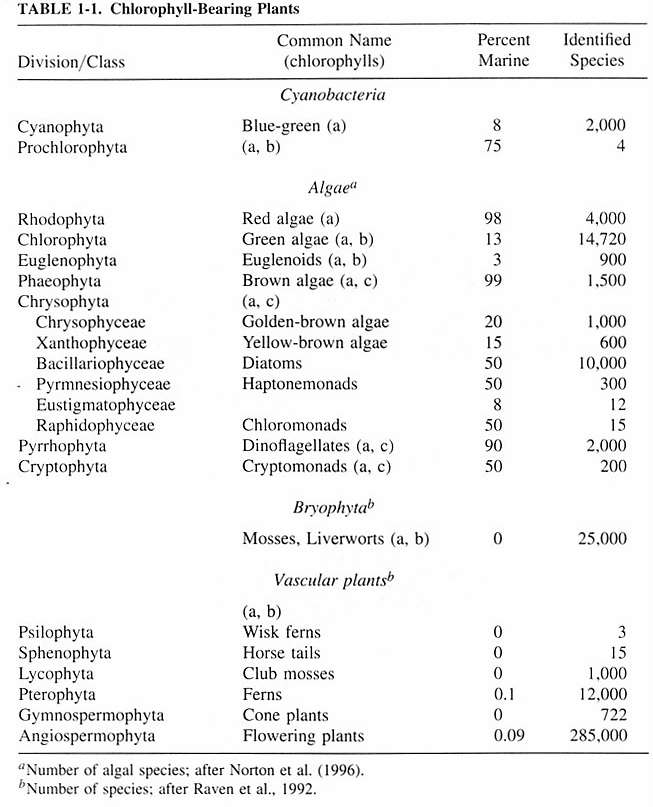
Phytoplankton belong to a diverse taxonomical
assemblage that had origins nearly 2 billion years ago. Some
phytoplankton are among the most evolutionarily ancient organisms
on earth, while others, such as the diatoms, have evolved
relatively recently. Most current taxonomical sources recognize
five Kingdoms: Monera (the bacteria and blue-green algae),
Protista (protozoa, algae, and slime molds), Plantae (true
plants), Fungi, and Animalia. Phytoplankton are found in Monera
and Protista, and none are found in the plant kingdom Plantae.
Current classification may not incorporate the five kingdom
scheme and more information can be found at: http://tolweb.org/tree/phylogeny.html
Kingdom Monera, or the bacteria and bacteria-like
organisms, have about 2000 discovered species in marine environments,
although it is likely many, many more exist. This kingdom
is divided into two primary groups, the Archeobacteria and
the Eubacteria, with the latter group having members, the
Cyanobacteria and the Chloroxybacteria, that contain chlorophyll
a. For many years, it was thought that the blue-green algae,
or cyanobacteria, were exclusively freshwater species, until
the discovery of marine species in the late 1950's. It is
now known that there are large numbers of them in both the
marine benthos and in marine plankton, and they play a major
role in nitrogen fixation in the marine environment.
Kingdom Protista was a "catch-all"
of organisms grouped mainly by being generally simple unicellular
species that do not fit in the other Kingdoms. It really has
disappeared from recent (last 5-10 years) treatments. Most
of the algae are considered to be part of this Kingdom today,
having been assigned mainly in the past to the Kingdom Plantae.
Even the macroalgae are largely considered to be in Protista,
and not Plantae, with a few somewhat disputed exceptions.
Phytoplankton are considered to be algae, and are not plants.
Algae are typically separated by the types of their photosynthetic
pigments, storage products, chloroplast structure, cellular
features, cell wall structure and composition, flagella (if
present), types of cell division, and life history traits.
Protist phytoplankton, with some exceptions, belong predominantly
to the divisions Chrysophyta, Pyrrhophyta, Euglenophyta and
Cryptophyta. There are none from the red or brown algae divisions
(Rhodophyta and Phaeophyta).
 From
Dawes (1998)
From
Dawes (1998)
Most phytoplankton are unicellular, either
solitary or colonial, although some may be multicellular and
filamentous. About 5000 species are currently described, but
estimates of 100,000 species of diatoms (based mainly on freshwater
forms) would indicate that the marine environment is, to put
it mildly, hugely unexplored. Phytoplankton are comprised
primarily of diatoms, dinoflagellates, coccolithophorids,
cyanobacteria, and other flagellates. Phytoplankton are found
in bodies of water ranging in size from small puddles, formed
after a rain, to the oceans. It may seem confusing that many
of these same organisms may normally live on sediments or
surfaces and become suspended by water turbulence; however,
these sedentary forms are not usually properly considered
true phytoplankton. Furthermore, some species have life cycles
that include a dormant or encysted stage that can last for
extended periods of time (up to years), and only part of their
life is planktonic. Most phytoplanktors are motile, with the
most motile being dinoflagellates that have swimming speeds
of 50-500 µm/sec. They are grouped according to cell
size and are often counted by measuring the amount of chlorophyll
in the water, rather than by actually sorting and counting
the cells, although the latter may be done for some studies..
The majority of phytoplankton, in numbers and biomass, can
be considered an autotrophic part of the microbial community
since they are in the smallest planktonic size classes; the
picoplankton and nanoplankton, also known as ultraplankton.
|
Plankton
type
Size in microns (mm)
|
|
Picoplankton
|
0.2
- 2.0
|
|
Nanoplankton
|
2.0
- 20
|
|
Microplankton
|
20
- 200
|
|
Mesoplankton
|
200-
2000
|
|
Macroplankton
|
>
2000
|
The picophytoplankton, perhaps the most
important component of the phytoplankton in terms of their
abundance and global ecological role, were only discovered
about 25 years ago (Johnson and Sieburth 1979). In fact, the
larger sizes, such as the microplankton seem only able to
develop when nutrient levels are above those required by the
pico- and nanoplankton. Picophytoplankton, comprised mainly
of prokaryotes including bacteria, cyanobacteria, and prochlorophytes,
as well as protist forms, can account for 50% or more of the
primary production of oceanic waters. A recent study has shown
that a single species of prochlorophyte, Prochlorococcus
marinus that occurs to a depth of 250m in the subtropical
and tropical oceans, can account for more than 50% of the
total chlorophyll-a in the central Pacific Ocean! (Suzuki
et al. 1995). Nannochloropsis, a genus of marine picophytoplankton
having cells smaller than 0.2 µm, is commonly employed
in "phytoplankton" products for marine aquariums.
This genus also is capable of forming dormant stages resulting
in forms where chilling can allow darkness tolerance for up
to 24 weeks (Antia and Cheng 1970). This is a relatively rare
trait that makes it an excellent candidate for live culture
products such as DT's phytoplankton™.
There is a great diversity of shape and
form in phytoplankton, and dinoflagellates and diatoms are
quite "famous" for these attributes. It is thought
that the various shapes are predominantly adaptations related
to their suspension in water. Generally, phytoplankton are
denser than water, having silica, cellulose and/or carbonate
components, and tend to sink. Various modes exist whereby
these tiny cells may remain as drifters in currents, rather
than sinking to the bottom. Some remain easily suspended because
of their diminutive size. Others have shapes that alter the
hydrodynamic forces and tend to give them "lift"
or "resistance." Some have gliding, flexing, or
active swimming behaviors, while still others have utilized
cellular components filled with gas or positively buoyant
material.
It is generally true that phytoplankton
use sunlight, carbon dioxide, nutrients and trace minerals
as requirements for their existence. However, a large component
of them, notably flagellates, can have pronounced heterotrophic
qualities; they can engulf, build up and store dissolved or
particulate organic material, and in many cases may be predatory.
It is important for phytoplankton to remain suspended in order
that they are able to obtain adequate light to supplement
scarce oceanic nutrients. Picophytoplankton, for instance,
have a negligible sinking rate, and their tiny size allows
for the most efficient diffusion of nutrients into and out
of the cell. Diatoms, in contrast, may depend on sinking resulting
from their density and size to force conduction and lower
the diffusive boundary layer surrounding them. Swimming behavior
also accomplishes this, especially when coupled with positive
phototaxis, or swimming toward light, such as seen in some
of the flagellates. Positive buoyancy, as found in many cyanobacteria,
also accomplishes higher nutrient uptake, but movement occurs
upwards instead of downwards.
Phytoplankton are largely limited to the
photic zone; an area from the water surface down to the point,
called the compensation depth (or critical depth), where the
energy production of phytoplankton by photosynthesis matches
their energy destruction by respiration. Even so, some phytoplankton
are able to exist short to rather long durations without light,
and can exist far below the photic zone. As might be expected,
since the zooxanthellae of corals are dinoflagellate algae
acting as "captive phytoplankton," the responses
of phytoplankton to light irradiance follow some similar patterns.
In general, they can modify their photosynthetic and associated
pigment concentrations, plastid size, thylakoid density, number
of photosynthetic antennae, or production of light shielding
compounds (mycosporine-like amino acids, or MAA's).
|
Division class:
|
Chlorophylls:
a b c1 c2 |
Phyco-
erythrin |
Phyco-
cyanin |
Allo-
phycocyanin |
ß-carotene |
Major
xanthophylls |
| Cyanophyta
Cyanophyceae (Cyanobacteria) |
+ |
+
|
+
|
+
|
+
|
Myxoxanthin
|
Pyrrhophyta
Dinophyceae |
+ + |
|
|
|
+
|
Peridinin
|
Chrysophyta
Prymnesiophyceae
(Haptophyceae)
Chrysophyceae
Bacillariophyceae |
+ + +
+ + +
+ + + |
|
|
|
+
+
|
Fucoxanthin
Fucoxanthin
|
Cryptophyta
Cryptophyceae |
+ + |
+
|
+
|
|
|
Alloxanthin
|
Chlorophyta
Prasinophyceae
Chlorophyceae
(and higher plants) |
+ +
+ +
|
|
|
|
+
|
Lutein
|
|
Table
3 . The distribution of photosynthetic pigments
in the most common phytoplankton taxa (from Richardson
et al. 1983)
|
Phytoplankton can use swimming and/or positive
and negative buoyancy attributes to moderate their light environment.
In general, phytoplankton are species that prefer a reduced
light environment; both photosystem damage and photoinhibition
are common at higher irradiance levels, such as those found
in shallow oligotrophic waters like coral reefs. Generally,
dinoflagellates and cyanobacteria grow best at very low irradiance
levels and may be photoinhibited even at low levels. Diatoms
can tolerate much higher irradiance levels, but may not prefer
to be exposed to it. The green algae typically have the highest
photosynthetic compensation points of the phytoplankton and
can tolerate very high irradiance levels (Richardson et al.
1983).
Phytoplankton require a source of nitrogen
that they can utilize after direct uptake. Nitrogen is usually
taken up as ammonium-N, with nitrate and nitrite uptake also
possible. Phosphorous is preferentially taken up as phosphate,
although they can also utilize polyphosphate and organic phosphate
sources. In general, microphytoplankton populations increase
significantly in response to nitrogen and phosphorus enrichment,
but the smaller nano- and picophytoplankton populations do
not (Takahashi et al. 1982). Silicon is required and acquired
by diatoms and silicoflagellates. Additionally, organic nutrients
such as vitamins are required. Although phytoplankton are
autotrophic with respect to carbon production, many are auxotrophic
(requiring a substance beyond levels normally found in the
environment) with respect to vitamins. In a study of 400 clones
of phytoplankton, 44% required vitamin B12,
21% required thiamin and 4% needed biotin (Swift 1980). Vitamin
requirements were found to vary according to species, with
certain groups, such as diatoms, requiring different vitamins
than dinoflagellates. Nanomolar concentrations of trace elements
required usually include zinc, iron, copper and manganese.
Higher levels may be toxic (Huntsman and Sunda 1980). The
vitamins and trace metals are thought to be acquired by their
association with bacteria, through the death of other microalgae
and zooplankton, and in areas where such organic and inorganic
components are found in greater abundance, such as in coastal
zones or areas of higher sediment loading.
Phytoplankton distribution, while quite
consistent when considered on a global scale, tends to occur
in patches on smaller scales. This is believed to be mostly
a function of water motion, patchy distribution of nutrients,
and the presence of herbivory by zooplankton or other sessile
phytoplanktivores. Blooms also occur, usually in spring, and
often occur successionally. The successions are usually initially
diatoms, followed by coccolithophorids and then dinoflagellates.
This is thought to result from both increased metabolism in
warmer temperatures, a response to rising irradiance levels
coupled with reduced zooplanktivory. While bacterioplankton
outnumber phytoplankton by several orders of magnitude on
coral reefs, diatoms and naked dinoflagellates tend to be
the predominant larger forms, along with picophytoplankton
and nanoplankton such as Platymonas spp. Other studies
found seasonal variations on coral reefs with 70% of winter
phytoplankton composed of protist nano- and picophytoplankton,
while Synechococcus sp. cyanobacteria formed the majority
during the summer (Yahel et al. 1998).
Rapid responses by the phytoplankton create
a nutrient deficit during the summer and result in reduced
phytoplankton growth. Most notable are the "red tide"
blooms of cyanobacteria and dinoflagellates that can produce
seriously harmful toxins. In the tropics, these are usually
caused by Trichodesmium spp. cyanobacteria. However,
not all cyanobacterial or dinoflagellate species that produce
blooms produce toxins, and not all harmful algae blooms are
red. Here are some links for supplemental information on red
tides:
http://www.redtide.whoi.edu/hab/whathabs/whathabs.html
http://www.tpwd.state.tx.us/fish/recreat/redtide.htm
http://www.marinelab.sarasota.fl.us/~mhenry/WREDTIDE.phtml
http://www.nwfsc.noaa.gov/hab/
Ecologically, phytoplankton are the major
source of primary production in the ocean, and one of the
most important driving forces of global ecology. In fact,
phytoplankton production influences all life by being at the
lowest rings of the food chain, and even plays a role in global
climate. In terms of their growth and ecology, they are in
many cases most similar to bacteria. In fact, only bacteria
share such similarities in size, growth rate ecological tolerance,
and rapid response to nutrient enrichment.
As an aside, I am reminded of an advertisement
in the aquarium literature that uses the promotional headline,
"Nature uses algae, not bacteria, to filter water."
In fact, this may be more the case than we realize. For many
years, we have operated under an old assumption that nitrifying
bacteria are responsible for converting ammonia into nitrite,
and then nitrate. However, the rapid proliferation of algal
"blooms" and diatoms in newly established tanks
begs the question of whether the phytoplankton are not equally,
or perhaps even more, involved in tank "cycling."
The single celled algae have doubling times from a few doublings
per day (in the smaller, faster growing species) to once every
week or so for slower species. During red tides in the Puget
Sound region, dinoflagellates such as Gonyaulax can
double every 30 minutes or so. They are also able to directly
take up ammonia from the water column, thus "cycling
the water prior to the nitrification "cycle."
Despite their rapid response to elevated
nutrients, phytoplankton are not particularly important primary
producers, by themselves, in terms of efficiency. The reason
they are so important on a regional or global scale is simply
by virtue of the fact that the upper 200m of oceanic waters
is filled with phytoplankton and covers over 70% of the earth's
surface. One source (Dawes 1998) compares primary producers
in terms of carbon production (kg C m-2
y-1)
as follows, from most to least efficient: corals and large
seaweeds (0.5 - 2.5), benthic microalgae (0.2 - 2.0), salt
marsh grasses and seagrasses (0.4 - 1.5), mangroves (0.5 -
1.0), coastal phytoplankton (0.1 - 0.5) and oceanic phytoplankton
(0.2).
Grazing - The Big Question.
What eats phytoplankton? In the water column,
zooplankton are without question the primary consumers of
phytoplankton. Zooplankton grazers vary according the area
and the time of year, but include primarily ciliates, copepods,
amphipods, and tintinnids. Protozoans and some invertebrate
larvae are the primary consumers of nanoplankton with copepods
and amphipods consuming the larger phytoplankton size classes.
Seasonal cycles with zooplankton increasing following phytoplankton
blooms are well known (Harvey et al 1935, Frost 1980). However,
the efficiency of zooplankton feeding on phytoplankton can
be extended to encompass a diurnal cycle. Evidence for zooplankton
as influencing daily cycles of phytoplankton abundance comes
from Yahel et al. (1998), where it was found that phytoplankton
abundance was greatly reduced over the course of the night
when zooplankton are at their highest abundance.
Of perhaps the most interest to those keeping
reef aquaria is to know what non-planktonic grazers of phytoplankton
exist on coral reefs. Benthic grazers are mainly bivalves,
ascidians (tunicates), sponges and polychaetes. Other major
consumers are gastropods, crinoids, foraminiferans and soft
corals. Because more carbon is contained in the biomass of
phytoplankton than zooplankton (usually by at least an order
of magnitude), and the nutrients are often limited around
coral reefs, phytoplankton are an obviously important food
resource to many organisms. The community of phytoplankton
consumers on coral reefs can drop phytoplankton levels 15-65%
below adjacent open ocean waters (Yahel et al 1998). Further
support for their consumption comes from a concomitant increase
in the levels of phaeopigments, breakdown products of phytoplankton
and substances released by zooplankton.
While some studies have indicated that
some stony corals are capable of clearing phytoplankton from
the water, these experiments have not been rigorous (Wilkinson
et al. 1988, Szmant-Froelich and Pilson 1984, Sorokin 1981,
1995). Ingestion does not equate to digestion. The extent
to which phytoplankton contribute to stony coral nutrition
is unknown, but it is probably unlikely that phytoplankton
are an important food source for most stony corals. Among
those reported or suggested to clear or ingest phytoplankton
are: Acropora, Siderastrea, Montipora,
Porites, Astrangia and Tubastraea. Other
studies tend to directly refute these suggestions for all
but Astrangia and Porites. More directly, Goniopora
and Alveopora may have more herbivorous tendencies
(Peach unpublished thesis). Stony corals are generally not
well adapted to the sieve or filter type feeding that characterizes
the soft corals (Fabricius et al. 1995, 1998). They are, however,
well suited to the capture of zooplankton prey. I am sure
that future studies will examine potential roles of phytoplanktivory
in the Scleractinia in more detail. However, I think it safe
to assume that the number of stony corals that depend on phytoplankton
as a food source will be minimal, or that the relative contribution
of phytoplankton to their energy needs will be slight.
Big Question Number 2 - How Much Should
I Feed?
As I said, I am not fond of giving recommendations
for aquarium protocol. Rather, I would prefer simply to communicate
some information and let each individual work out the "rules"
that apply to their own circumstances. It is of paramount
importance to recognize that the biomass of potential grazers
in an aquarium is many times what it would be in the same
volume of water or surface area as the bottom of oceans or
on reefs, and also that the availability of water column borne
food is many times greater in the ocean than in an aquarium.
This creates an immediate and perhaps irresolvable dilemma.
It is also important to consider the types, sizes, and numbers
of potential consumers of phytoplankton in a given aquarium.
For example, a clam and soft coral tank with a large sponge
community and a refugium with many small crustaceans and a
deep sand bed with many polychaetes will have a vastly greater
uptake/consumption of phytoplankton than a bare bottom, no
refugium stony coral garden-type tank.
However, if one were to attempt to recreate
natural levels of phytoplankton in the aquarium, the following
chart may be of some use, especially if the density of cells
per phytoplankton product is known.
|
Organism
type
|
Density
of cells/ml water
|
|
Bacteria
|
106
|
|
Phototropic
picoplankton and nanoplanktonn
|
104
|
|
Nanoplanktonic
flagellates
|
103
|
|
Microphytoplankton
|
103
|
|
Viral
particles
|
108
|
I mention bacteria and viruses in the chart
for two reasons. First, to call attention to an upcoming part
in this series on the food value of bacteria. Second, because
viral infection accounts for between 30-60% of mortality in
bacteria and cyanobacteria in the ocean (Proctor and Fuhrman
1990), and it is suggested that similar mortality figures
for phytoplankton are likely, especially given the abundance
of viral particles in the water. Third, and perhaps most importantly,
is the almost ubiquitous interaction between bacteria and
phytoplankton. Phytoplankton release dissolved organic substances
and bacteria utilize them as nutrient sources. Most phytoplankton
cells, especially large ones, are coupled nearly continuously
with coatings of bacteria. The bacteria are apparently both
beneficial and detrimental in that they provide breakdown
products able to be utilized by the phytoplankton, but also
increase the diffusive boundary layer whereby the phytoplankton
would ordinarily be able to directly uptake nutrients from
the water.
In conclusion, phytoplankton are hugely
abundant in numbers and biomass and are present at levels
that make them among the most ecologically important groups
on the planet. Diversity and biomass is likely to be higher
near coastal environments and in temperate environments rather
than in oceanic or oligotrophic tropical waters like coral
reefs. They constitute an important trophic resource to zooplankton
and many sessile benthic invertebrates, perhaps ironically
not those to which a majority of aquarists apparently expect
to see direct benefits of phytoplankton additions. In regards
to aquariums, I offer the following bulleted list of suggestions
and comments regarding phytoplankton in reef aquariums:
-
The amounts of phytoplankton present
in reef aquariums are not known but are probably considerable.
However, they are also probably rapidly removed by grazing
and export devices.
-
Similarly, the amounts required to
produce an equivalent level to sustained natural seawater
levels is not known, and is probably highly dependent
on individual differences in tank stocking, but is likely
a considerable amount.
-
Algae "blooms" in aquariums
are normal, and follow seasonal patterns that are apparently
similar to those found in the wild. As in the wild, grazing
or nutrient conditions probably change rapidly to limit
blooms to rather short durations.
-
Many of the phytoplankton are species
not normally considered or deemed desirable, including
diatoms, dinoflagellates, and cyanobacteria. Yet, just
as they are important and functional constituents of wild
phytoplankton populations, so they should be in aquariums.
-
Not all phytoplankton will likely be
beneficial in aquariums. Many species produce toxins that
can be harmful to tank inhabitants. Common evidence of
this is found in coral deaths involving cyanobacterial
blooms and snail and echinoderm deaths during dinoflagellate
blooms. However, not all dinoflagellates or cyanobacteria
necessarily have these toxins or produce these results
in aquariums.
-
"Psuedo-phytoplankton" is
probably available in tanks in significant amounts when
substrate associated algae are put into circulation by
strong water flow or during tank glass scraping.
-
In order to maximally benefit the largest
number of potential tank inhabitants that are phytoplankton
consumers, it is probably wise to allow for the occurrence
of similar ratios of various size classes as found in
the wild. To my knowledge, despite the numbers of available
products, relatively few variations in size classes are
available, and thus supplementation with a certain size
class will probably not benefit large groups or organisms
incapable of feeding on them.
-
Supplements using living cultures or
products containing living cells are probably far more
beneficial than those made of dead cells. Dead cells are,
for all practical purposes, decomposing particulate material
that, while potentially useful as a food source, do not
offer the benefits of living cells as described in the
article.
To end this article in the continuing series
on coral reef food, I offer the following list of "famous"
phytoplankton:
Symbiodinium spp. - the
symbiotic algae of corals, clams, and other organisms are
dinoflagellate protist phytoplankton.
Pfiesteria
piscicida - dinoflagellate ambush predator responsible
for fish kills by micropredation.
Trichodesmium
spp. - one of several cyanobacterial phytoplankton causing
"red tides".
Gambierdiscus
toxicus - one of several dinoflagellates responsible for
ciguatera poisoning.
Ostreobium
quecketii - the microalgae found living in the extremely
low light of coral skeletons below the living tissue.
Nannochloropsis
oculata - the primary phytoplankton of aquarium phytoplankton
products.
Pseudo-nitzschia
australis - newly discovered toxic diatom that accumulates
in fish and shellfish and can cause brain damage in low amounts.
Links to
Part
1, Part
2 , Part
4, Part
5, Part
6, Part
7
|