|
Introduction
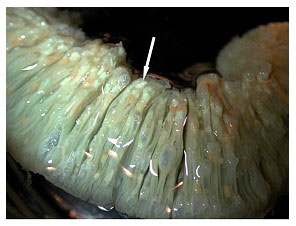
For many years in the late 1980s and throughout much of the 1990s, elegance corals (Catalaphyllia jardinei Wells 1971) were among the easiest corals to keep in aquaria. These beautiful corals have large fluorescent green polyps with long tentacles extended during the day, obviously conspicuous from any other coral. The fluorescent qualities of Catalaphyllia under actinic or blue light are breathtaking; bright fluorescent green and white striations appear on its oral disk, often with contrasting blue, orange or purple-tipped tentacles. It is little wonder that they are desirable species for aquariums.
Normally, Catalaphyllia are very willing feeders and the nematocysts present in their tentacles have the same “sticky” quality as many anemones. Their tissue is almost fully retractable, but usually remains partially expanded at night. The degree to which these corals can expand, coupled with their strong stinging cells, makes any nearby coral susceptible to severe damage. Their tissue can be easily punctured or torn when fully expanded, and care should be taken never to remove an expanded colony from water without causing its polyps to withdraw by fanning them underwater.
As a member of the family Euphyliidae, the skeleton of Catalaphyllia is almost identical to the flabello-meandroid species of Euphyllia. Like Trachyphyllia, Catalaphyllia often are temporarily attached to substrate while young, but often break away to become free living when mature. They also can remain attached to reef substrate where they tend to grow larger, with colonies more meandroid in growth form. When free-living, life in the soft substrates tends to impose an upper size limit on the colonies.
These corals occasionally “bail out” of their skeletons (Borneman 2006), and may do so in response to poor water parameters or from other undetermined causes or stresses (Figure 1). This is not a normal form of reproduction, but seems to be an “escape” reaction and invariably leads to the colony’s loss. The unattached colony of polyps can live for a long time with good water quality and care but, to my knowledge, never again produces a skeleton, despite perhaps maintaining the potential to do so through the calicoblastic epithelium's cellular calcification mechanisms. Invariably, the inability to keep a detached colony affixed in the aquarium results in its accidental demise. I am not aware of any long-term survival of an elegance colony that has become detached from its skeleton, or any that have again begun to produce a skeleton.
 |
Asexual reproduction is uncommon, but small buds, similar to those of Euphyllia, occasionally form along the skeleton's margin, and these can detach or be removed to produce daughter colonies. The parent colonies can be cut into fragments with high survivorship using an electric “wet” tile saw filled with seawater. The colony can be sawed straight into halves or even smaller pieces - as small as an inch across. The colony in my tank (Figure 2) resulted from an edge fragment cut from a larger colony that I owned. The fragment was about 3cm long, and I even cut that in half using a wet saw, and used the other half for histological work. The polyp tissue is deeply embedded in the skeleton and using hammer and chisel, cutters, Dremel tools or other less precise cutting methods is generally very destructive to the tissue, and results in poor survivorship of the fragments produced. Sexual reproduction is rare and virtually unknown, even in the wild. Colonies may be either brooders or hermaphroditic broadcast spawners, releasing large egg/sperm bundles (Borneman 2006). Both of these reproductive methods have been observed in aquariums (Figure 3) and it is unknown why, where or under what conditions these different methods are utilized.
 |
Catalaphyllia are found in the central Indo-Pacific from northern Australia through the Philippines and Indonesia, southeast Asia and southern Japan. They also are found in the western Indian Ocean off the east coast of Africa. The species can be locally common to abundant in protected turbid or lagoon areas with soft or muddy bottoms (although they may be found in other areas), and in inter-reef areas where they commonly share space with seagrasses. They are rarely common throughout their range and occur in patchy distributions where they may be abundant. Patchy abundant areas of free-living colonies found sporadically across habitats are typical of brooding species.
Virtually all Catalaphyllia in the aquarium trade come from Indonesia, with a small number arriving from a few southeast Asian nations with more recent and largely unregulated collection operations. Australia has recently begun to export the species as well. Indonesian quotas for Catalaphyllia are generally in the tens of thousands per year, although these quotas have decreased in recent years and can vary. In 2001 we undertook surveys to begin assessing the aquarium trade's impact on species put under trade suspension in the EU (Bruckner and Borneman 2006; Bruckner 2002).
Catalaphyllia's past reputation as being easy to maintain in aquariums for many years makes sense given its broad distribution range described above. Found in waters deep (>40m), shallow (<1m), turbid or clear, attached on hard patch reef substrate and free-living on soft substrates, this species occurs in greatly diverse habitats. Beginning sometime in the late 1990s, however, some specimens entering the trade were doomed by a condition that had no known cause or cure. Over time, virtually all specimens in the trade showed signs of this lethal condition, which causes the colony's oral disk to swell, with a fringe of unexpanded tentacles (Figure 4). In many cases, a white opaque mucus-like web is present (Figure 5). Feeding responses decline and tentacles are no longer “sticky” or able to easily capture prey items, even non-motile particulate material. The coral's tissue eventually shrinks, and the colony dies despite all manners of experimental intervention that have been attempted (Figures 6.1 and 6.2). Several authors in the aquarium hobby have written about this condition and proposed theories of why these corals currently exhibit such poor survival. On Internet message boards and magazines, there has been much speculation as to why this species no longer thrives in aquariums, and along with these speculations putative “cures” have been proposed. None of this speculation, however, has any good evidence - in theory or practice - to support it. Because this malady is characterized by a distinct set of signs, it meets the definition of a disease, so I will refer to it as elegance coral syndrome (ECS) until its etiology is better understood and a more appropriate name for the condition(s) becomes appropriate. I have never heard of any C. jardinei that has been established in an aquarium contracting ECS without being in direct or indirect contact with another specimen with ECS. Although they may die of many causes, established C. jardinei remain exceptionally hardy corals in captive systems that do not contract ECS. Several years ago I undertook a project to determine the cause(s) of the high mortality rates that ECS caused in C. jardinei within the aquarium trade, coupled with fieldwork and other reporting methods.
 |
 |
Materials and Methods
Field Component
Surveys were conducted at 11 coral collection sites throughout the Spermonde archipelago where Catalaphyllia jardinei was reported to occur and where most Catalaphyllia are collected. Transects, manta-tows and simple searching by snorkel and scuba were all performed to assess populations. Interviews were conducted with coral collectors, exporters and members of the coral trade group AKKII. Collected corals were counted, examined and measured at holding stations in the field and at several large export facilities, and any specimens with ECS were noted.
Reporting Component
Contacts and interviews were made with divers, scientists, collectors, aquarists and coral trade personnel in Vietnam, India, Fiji, Australia and the Philippines. Reports were also made by aquarists in Europe on Internet message boards and during my trips to several countries in Europe. The primary purpose of the interviews and reports was to determine if and to what degree Catalaphyllia was affected by ECS syndrome in the wild and in aquariums. I also wanted to find out the degree to which the corals were being kept in aquariums and with what degree of success, with regard to colonies showing signs of ECS. Finally, I wanted to know if C. jardinei were obtained locally or were imported, and from where.
Collection of Samples
Catalaphyllia specimens were acquired from various sources, both healthy and affected with ECS. I directly requested and obtained samples from aquarists of C. jardinei that showed signs of ECS. I also requested various other materials and documentation according to previously described protocols (Borneman 2004). In all, 79 usable specimens were obtained; 70 showed signs of ECS or other pathologies, seven were apparently healthy and showed no signs of ECS and two did not have ECS and had been maintained in captivity for over five years. In total, 75 ECS-affected colonies were used for histological analyses, and two fragments of a healthy colony were available as controls. I was unable to obtain other apparently healthy colonies as controls (see discussion).
Disease Description
Five specimens of C. jardinei in the initial stages of ECS were placed into quarantine tanks and observed for up to several weeks. The signs of the disease's progression were noted and documented. Before complete tissue mortality occurred, the specimens were fixed as described below.
Contagion Experiment
Five specimens of C. jardinei that did not show signs of ECS were placed in close proximity to a specimen with ECS to determine if the apparently healthy corals could contract ECS without direct contact. These healthy specimens were specially ordered from a collector in Indonesia (two collected in Jakarta, two collected in Sulawesi and one collected in Sumatra), and were to be collected and prevented from coming in contact with any other Catalaphyllia or aquarium water, remaining in their own collection bag and only being reoxygenated during shipping. In a second trial, a specimen with ECS was placed into a multi-tank system sharing the same water with a specimen that did not have ECS and that was located in a separate tank. Other coral species in the contagion aquariums were also observed for any signs of stress or disease from the ECS-affected corals.
Specimen Preparation
Each living specimen was photographed upon arrival, placed into an aquarium and photographed again after acclimation and tissue expansion, unless the coral was in an advanced disease state, in which case it was photographed and immediately fixed for histology. Because the corals' polyps can completely withdraw into their skeleton, it was necessary to allow the corals to expand under water in an aquarium to assess their condition. After it was determined that the gross signs of disease were consistent with ECS, the corals were removed from the aquarium and fixed. The aquarium water was sterilized by pouring a large amount of bleach into it, and then disposed of by pouring it into a drain that led to on-site water treatment. This was not done in the contagion experiments, in which corals were placed into normal reef aquarium environments where all aquarium water remained in circulation. Some specimens were fixed prior to shipping after the donator had photographed them and I had determined that they showed signs of ECS. The corals were fixed according to previously described protocols. Depending on the fixative used (alcohol or formalin), the corals were either stored or post-fixed in zinc formalin (Z-Fix, Anatech, Ltd.).
Live corals were fixed for 48-72 hours, depending on their size and amount of tissue, in zinc formalin (Z-Fix, Anatech Ltd). They then were decalcified using acidified EDTA until all carbonate skeleton was removed. Notes were made of any anomalous organic material and organisms that remained after decalcification, such as Lithophaga sp. bivalves, polychaetes, sponges, algal pieces and fungal filaments. Aliquots of stirred decalcification liquid were examined under a microscope for the presence of microorganisms that might be consistent with ECS-affected corals. Decalcified tissues were rinsed in deionized water and then placed into 70% ethanol for storage and shipping.
The corals were recorded and sent along with required forms to the International Registry of Coral Pathology (IRCP) in Bethesda, MD, where histological preparations were made. Gross photographs of whole tissues were made and the corals were processed and sectioned into 6µm slices in the vertical and horizontal plane, and mounted onto glass slides. The slides were stained with paraldehyde fuchsin (PAF), hematoxylin and eosin (H&E), as well as three unstained mounts for various other stains. I subsampled several highly characteristic samples and used gram stain, TUNEL stain, iodine, trypan blue and saffranin to probe for other potential histological characteristics associated with the diseased tissue.
Slides were examined under a dissecting scope (Olympus Wild) for general tissue condition and the presence of large potential etiological agents. Stained slides were examined under normal and phase contrast microscopy (Leitz Dialux 20) and fluorescent microscopy (Olympus AX70 fitted with a DAPI filter and Magnafire software).
Results
Field Component
In all geographically surveyed areas, Catalaphyllia jardinei was found to be rare (Tables 1 and 2). It occurred in attached and free-living forms in shallow and deep water, in silted areas and on soft bottoms.
| Site |
Habitat |
Depth |
Genera |
Mean diameter |
corals/m2 |
| 1. P. Kuricaddi # |
soft -bottom |
1-3 |
23 |
8.4 cm |
0.3 |
| 2. P. Kuricaddi # |
soft -bottom |
3-4 |
8 |
9.8 |
0.2 |
| 3. Bone Batang (NW) * |
soft-bottom |
30 |
8 |
13.5 |
4.7 |
| 4. Bone Batang (W) * |
soft-bottom |
32 |
5 |
15.2 |
15.5 |
| 5. Bone Malonjo SE |
soft -bottom |
35 |
6 |
7.5 |
2.0 |
| 6. Pulua Baranglompo |
patch reef |
15 |
27 |
23.4 |
4.0 |
| 7. Bone Lola |
patch reef |
15 |
31 |
19.8 |
5.8 |
| 8. Bone Malonjo |
patch reef |
26 |
20 |
8.6 |
3.0 |
| 9. P. Kudingarengkeke |
patch reef |
15-20 |
53 |
18.9 |
9.4 |
| 10. Samalona |
fringing reef |
9 |
39 |
18.6 |
7.5 |
| 11. Barangcaddi |
fringing reef |
5-10 |
51 |
14.4 |
6.8 |
|
|
Table 1. Population dynamics of EU-suspended scleractinian corals in Spermonde Archipelago, Sulawesi. All species examined along transects are pooled. |
| Coral species |
Density
(number/m2) |
Area (km2) |
Total population |
% of stock available |
Quota |
% of harvest |
| B. wellsi |
0.05 |
160 |
8,000 |
100% |
2,500 |
31.0% |
| C. jardinei |
0.03-0.05 |
260 |
10,400 |
90% |
10,000 |
100% |
| C. lacrymalis |
0-0.1 |
6,240 |
166,000 |
70% |
2,500 |
2.2% |
| E. ancora |
0.1-0.2 |
1,760 |
750,000 |
85% |
6,000 |
0.9% |
| E. cristata |
0-0.01 |
1,760 |
10,000 |
85% |
7,000 |
90.0% |
| E. divisa |
0-0.17 |
1,760 |
72,000 |
85% |
0 |
???? |
| E. glabrescens |
0.03-0.9 |
1,760 |
900,000 |
85% |
9,000 |
0.9% |
| Hydnophora spp. |
0.1 |
1,760 |
176,000 |
80% |
4,000 |
2.8% |
| N. turbida |
5.0 |
160 |
800,000 |
100% |
3,000 |
0.4% |
| Plerogyra spp. |
0.08-0.19 |
1,760 |
227,000 |
63% |
6,000 |
4.2% |
| T. geoffroyi |
0.1-0.28 |
4,740 |
627,000 |
83% |
11,000 |
1.9% |
|
|
Table 2. Estimated abundance of each taxa and potential amount of harvest in the Spermonde Archipelago, South Sulawesi. The percent of stock available is the total percent of colonies within the legal size range as established by Indonesia. The percent of the population in Sulawesi that would be removed each year assumes that collectors take 100% of the allocated quota. |
During our time with Indonesian coral collectors off the large collection areas of Makassar, Sulawesi, they assured us that Catalaphyllia would be very common and that they were collected from many places. In contrast, they were quite uncommon at all the sites where we were taken, and the accounts given by coral collectors seemed to vastly overstate their abundance. We did find colonies at several different locations, however, and, like Euphyllia, they occurred in very different habitats. We also found them to adopt quite distinct morphotypes depending on the area of collection (Borneman 2002). Collection sites in deep water (>40m), where the most colorful red and green Trachyphyllia were also collected, were the areas where we saw the most harvesting of Catalaphyllia. This is a very low-light environment with a sand and silt seafloor and no hard substrate. It is sparsely populated by free-living corals, macroalgae and cyanobacterial mats. Here, Catalaphyllia were found as small, free-living colonies that are generally the size and shape of the vast majority seen in aquarium stores. However, every Catalaphyllia seen - and every Catalaphyllia collected - from this area had purple tentacle tips. None of the Catalaphyllia at any other site we visited had this desirable color characteristic. Veron (2000) notes, however, that the presence of colored tips is a common color pattern throughout this species' range.
In contrast, the very few colonies of Catalaphyllia we found in shallow fringing patch reefs grew on the hard reef substrate and were much larger, adopting a significantly developed flabello-meandroid growth form with a fluorescent green and white striated oral disk, and without colored tentacle tips. Such colonies, being attached, would be removed by hammer and chisel or crowbar for collection, and would have very white and clearly broken skeletal bases, rather than the discolored, cone-shaped or unbroken bases typical of free-living colonies at all other sites.
Another collection area was a nearshore, silty, shallow area where dull-colored Trachyphyllia were collected. It was also reported to be a prime area of Catalaphyllia collection. We found the area devoid of many Catalaphyllia colonies, so we must assume either sporadic occurrence, low natural density or overcollection. Like the deepwater habitat, Catalaphyllia collected here were free-living and never attached. They were found nearly buried in deep fine silt and were, also like the deepwater colonies, small and apparently size-limited by the substrate. More notably, the thick brown silt had discolored their skeletons from white to a dingy brown. Their coloration was drabber, being brownish with muted green-brown oral disks and tentacles.
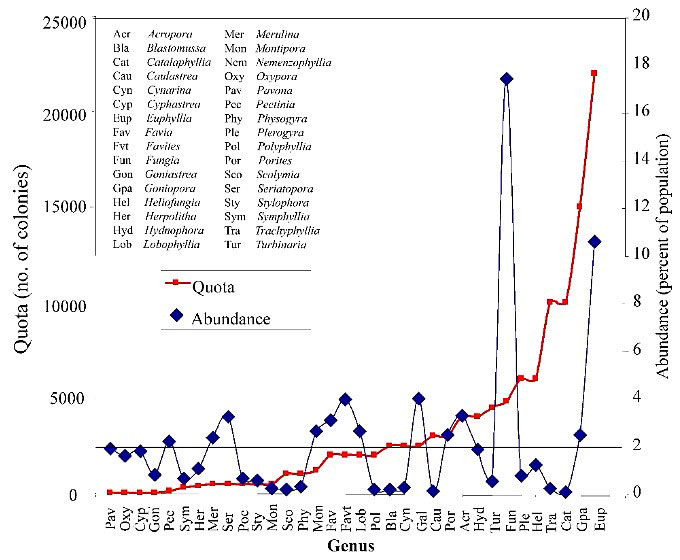
An area has been reported in shallow seagrass where colonies are abundant (Raymakers 2001), although Catalaphyllia were not documented in any seagrass areas we examined. With few exceptions, these corals were found to be extremely rare to absent at all collection sites we visited. In the soft-bottomed sites where we found the most colonies, the species was free-living and occurred at a density of 0.05 colonies/m2 (Bruckner and Borneman 2006). The patch reef contained primarily large attached colonies with meandroid growth that exceeded the preferred size for the trade. Given that Catalpahyllia are still reported from countries in regions with no coral collection for the aquarium trade, and in habitats where they were no longer found in Indonesian collecting areas, overcollection seems to be a significant concern and a logical explanation for the low abundance of the species we found in the areas where they are reported to (and should) occur. Unless the species does still occur in shallow grassbeds, an annual harvest quota of 10,000 and the low abundance of colonies we found, extrapolated to the entire collection area, would eliminate the entire population of colonies within the preferred size class (Figure 7). Little is known of this species' life history traits (recruitment rates or reproduction) in the wild, so sustainable collection levels are almost impossible to determine. Therefore, solely to conserve the species in areas where collection occurs, aquarists should avoid purchasing wild-collected Catalaphyllia until the species' sustainability is more rigorously assessed. Unfortunately, captive-propagated specimens are extremely uncommon.
 |
In no case did we find any colony exhibiting signs of ECS in the field. Similarly, in the holding facility where corals are kept following collection and prior to reaching the export facility, no corals exhibited signs of ECS (Figure 8). Several large exporters held C. jardinei in large concrete pools that contained only C. jardinei, and several dozen to several hundred corals were maintained with tight spacing and contact between most colonies. The total water volume per number of corals was low, and water changes were performed weekly by trucking in seawater. In one export facility containing hundreds of C. jardinei, we found a single specimen showing signs of ECS (Figure 9).
Reporting Component
My interviews with people in resource countries where Catalaphyllia are found naturally and/or kept in aquariums indicated that no one had seen ECS in the field or in aquarium specimens. This was particularly notable in Australia, where these corals are relatively common, collection is monitored and managed, and there are many aquarists. It is also notable that Australia forbids the import of this species from other areas. In Europe, ECS was reported in countries that allow the import of C. jardinei, but the incidence by report was lower than the number in the United States. European countries receive their corals from many of the same sources that the United States does; and Indonesia is the primary exporter of Catalaphyllia to both Europe and the United States, with the vast majority destined for the United States.
Collection of Samples
Despite describing a rigorous sample collection, handling and shipping protocol, many specimens sent to me were problematic. In some cases, the protocol was not followed exactly, bags leaked and fixatives were not added as required. Post-fixation was attempted in order to improve fixation for histology in such cases. In several shipments, the live coral died in transit, and these were excluded from the study (Figure 10). Other forms of sampling, including mucus swabs and photographs, and including the documentation requested, was not provided. In samples I obtained, corals were carefully handled and maintained prior to fixation. In some of the samples sent, fixatives varied; several ethanol preparations, formaldehyde and isopropyl alcohol were all used. All samples I obtained were fixed in zinc formalin, which also was used as the post-fix for corals sent in other fixatives.
Contagion Experiment
Experiment 1
Five corals individually collected and bagged were sent to a coral culture facility in Houston. All of them appeared healthy upon arrival. Three were placed in close proximity to, but not in contact with, each other, into a single large culture system housing multiple corals and containing multiple connected tanks. This system had not previously held C. jardinei with ECS. One (Jakarta 1) was placed into a separate smaller reef aquarium stocked with multiple corals, fishes and invertebrates that did not contain, and had never contained, C. jardinei. The last (Sulawesi 2) was placed in a similar, but slightly larger, reef aquarium containing a different mix of species (Figure 11). No corals in any of the tanks showed signs of any disease, and all species appeared grossly healthy and were growing. Water quality in all tanks was excellent as measured by standard aquarium water quality parameters, which were within oceanic levels. Lighting varied from intense 400-watt metal halides (800 PAR at the coral) to low full spectrum fluorescent (60 PAR), roughly representing the range of lighting colonies are exposed to in the field.
 |
 |
Within several days of exposure to a specimen with ECS, one of the C. jardinei specimens from Jakarta (Jakarta 2) in the culture system began showing signs of ECS. Within three days, this stunning red C. jardinei (Sumatra) began showing signs of hyperinflation of its oral disk, and shrunken tentacles (Figure 12 above). Within one week, another C. jardinei (Sulawesi 1) also began to show signs of ECS. Jakarta #2 was removed from the system and fixed for histology (Figure 13). Sulawesi 1 and Sumatra were removed one week and 10 days later as ECS progressed, and were fixed. Jakarta 1 and Sulawesi 2, each in individual tanks, continued to survive and appeared healthy for several months. These were planned to be used as healthy control specimens, but the facility sold them to customers without my knowledge, so they were unavailable for the study.
Experiment 2
During the collection of samples, I received many within days or weeks of each other and ran out of space and tanks to hold all of them. I received one specimen with ECS late in the afternoon and planned to take it to my university's lab the following morning. In an adjacent tank sharing the same water was a C. jardinei that had been in my tank for six years. The coral with ECS was removed and fixed the following morning. One week later, however, the six-year established specimen in my tank showed signs of ECS. I attempted numerous experimental treatments with various antibiotics and anti-parasitics, none of which had any effect. A two-hour nitrofurazone bath, which had been anecdotally reported to help with this condition, fouled the water in the treatment aquarium and killed the coral. As a result, this coral was unavailable as either a healthy control or an infected specimen.
Gross Description of the Disease Process
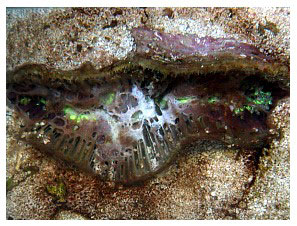
Catalaphyllia affected with ECS show a progression of both consistent and inconsistent signs. The first signs are often, but not always, an aperiodic expansion and contraction of the colony and excessive mucus production that streams from the oral disk’s surface. This is followed by the characteristic signs of an expanded oral disk and the progressive lack of extension of the polyps' tentacles, eventually being reduced to a fringe of small flaccid tentacles surrounding the disc (Figure 4). The coral's color almost always changes, with the bright fluorescent green oral disk becoming paler, and with an underlying opaque white to grey color (Figures 5 and 9). At this point, the colony is all but incapable of capturing food particles or prey, and the tentacles do not stick to prey items. In 68% of cases (n=48) in this study, a white or grey web of mucus forms a web-like covering across the oral disk, and appears to originate near the interface between the coral's tissue and skeleton (Figure 5). This second stage typically lasts from days to nearly a month. The coral then begins to recede and its oral disc becomes deflated. Tissue loss begins, usually near the lateral margins, moving inward toward the central polyps (Figures 6.1 and 6.2). The coral ceases to expand either its tentacles or its oral disk, and tissue loss progresses until the coral dies. Secondary infections may occur, but are neither common nor predictable (Figure 14).
 |
 |
One aspect of the gross histology is noteworthy. In 28% of the colonies, all of which displayed signs of the white/grey web-like material, opaque bodies were visible in the coral's tissues nearest the skeleton, that lay between the septa (Figure 15 above). These were examined microscopically and are discussed below. Some photos of the gross specimens are also shown (Figure 16).
Histology
The histology of C. jardinei affected with ECS is interesting. Both consistent and inconsistent elements appear. The latter category includes the presence of ciliates, other protists, what appear to be deposited eggs, fungal filaments and heavy bacterial mats at the coral’s epithelial surface (Figures 17 - 21). These are covered in the discussion section.
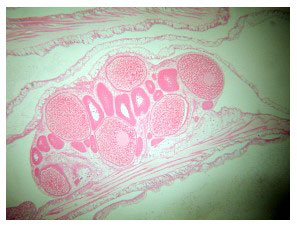
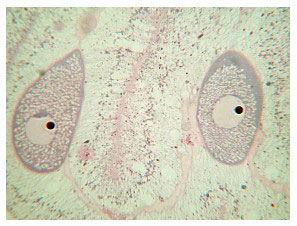
Five of the specimens in the study contained reproductive structures; eggs, ovaries and spermaries were all evident (Figure 21). The presence of developing embryos indicates that this species is a hermaphroditic brooder (Figure 22), although, as mentioned, egg sperm bundles have been documented being released, as well as tentacle brooding of planulae (Borneman 2006).
 |
 |
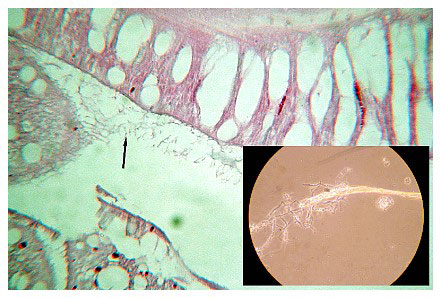
In many samples, fungal filaments were found attached to and sometimes penetrating the calicoblastic epithelium (Figure 23). This has been shown previously as a portal for microbial invasion. Polyps were dissected and the basal tissue's white opaque areas were removed, and the material was heat-fixed and examined under the microscope. Sections of the tissue and material were sent to Scripps Oceanographic Institute for confirmation, because the material resembled actinomycetes. The results showed that the material was dead and necrotic tissue with the appearance of occasional fungal filaments, and it did not test positive for actinomycetes.
 |
The consistent, anomalous histological feature of corals with ECS is the presence of gram negative bacterial aggregates throughout their tissues (Figure 24, black arrows). These aggregates vary in size and number according to how far along the disease has progressed. They resemble aggregates seen in other diseased corals (Santavy, pers. comm.), but their identity is not confirmed. The second most consistent histological anomaly is the appearance of green rods within both the intertissue space and the tissue (Figure 25). These rods appear to aggregate near, invade and inhabit the zooxanthellae once inside the tissue. Santavy (pers. comm.) suggested that these may be refractile objects, but high magnification shows them inside the zooxanthellae in patchy areas, or occasionally throughout the gastrodermal zooxanthellae. Many corals with these rods also show a reduced density of zooxanthellae in their tissue sections.
 |
 |
In terms of their tissues' condition, corals with ECS show a range of tissue degradation that correlates with the disease's progression. Early stage ECS sections have occasional gram negative aggregates and their tissue remains more or less intact. As the disease progresses, the tissue's integrity becomes increasingly compromised. One of the more notable aspects is that nematocysts become granulated and eventually are destroyed. The presence of the aggregates near the nematocysts is also notable. In late-stage ECS, the tissue's integrity is all but lost. Despite the coral tissue's appearing more or less intact in the living corals, histologically the cells are necrotic and disintegrating. The boundaries between epidermis and gastrodermis are progressively lost, and the mesoglea disappears. At this stage, many secondary invasions can occur. The tissue appears visually to be a mass of unconnected cells, zooxanthellae and cellular debris.
In terms of corals in which the green rods are present, zooxanthellae lose their normal fixed coloration and become green as they become filled with rods. These rods are present in about 50% of ECS affected corals. The discussion section below covers the rods' presence.
This study included only a single healthy control sample. It was sent to the IRCP for processing, but the sample was never processed and returned (discussed below). Before sending the sample, I fragmented a small piece, and that coral has remained in my system for several years now and has matured into a full-sized, healthy colony. I produced several squashes of tissue from its tentacles, oral disc and marginal sections to examine this apparently healthy coral for the aggregates or the rods, and none was found in any section.
Discussion
It is apparent from field work in Indonesia, and from reports in other countries where Catalaphyllia are found, that ECS is apparently a rare or patchy condition. It has not, to my knowledge, been reported in the field. I suspect it does occur to some extent, though, because of the single specimen seen in a Jakarta exporter’s tank. Corals are not held in these tanks for long before export, but it is possible that ECS is caused by land-based or human contamination, rather than being a field condition.
The apparent lack of ECS in other countries that do not import corals from Indonesia, or where people directly collect and place corals into aquariums, such as the Philippines and Australia, suggests that it this disease is caused by extraordinary conditions: either harvest from rare ECS affected colonies, from handling and storage or from inadequate reporting. However, the condition's epizootic nature, given the large numbers of C. jardinei exported from Indonesia and their resultant mortality in the United States, which imports the vast majority of those collected specimens, suggests that some assumptions can be made based on the results presented here. Not all Catalaphyllia in the trade show signs of this condition, although surveys of regional and national stores (n=15) indicate a prevalence rate of 83% using diagnosis by gross signs of disease. Specimens that appear normal and healthy are occasionally found in stores, although aquarists' reports suggest that many of these became diseased soon after purchase, despite not being in contact with other C. jardinei corals. This suggests that those corals acquired the condition prior to their introduction into the aquarium, but does not exclude the possibility that they acquired it in the aquarium. This seems unlikely because no long-term captive elegance corals have been reported to contract ECS, and ECS generally occurs very soon (within days to weeks) after placement into an aquarium.
The condition is contagious, and I have caused healthy corals to show signs of the disease within days of exposure by placing them adjacently to diseased specimens, or by placing diseased specimens and healthy specimens in different tanks that share the same water. These contagion trials indicate a high degree of infectivity by an unknown agent, and that it is waterborne and does not require direct contact. The time from exposure to grossly observed signs of disease ranges from a few days to a few weeks. The disease appears to be specific only to Catalaphyllia because no other corals to date (over 20 genera and 50 species with multiple genotypes of each species, including the most closely related family members of Euphyliidae) have shown signs of the condition upon exposure to diseased Catalaphyllia.
Catalaphyllia exist in a wide range of environments and appear to be unsustainably collected in Indonesia. Early in the reefkeeping hobby's history, most specimens had a distinct appearance and a white skeleton consistent with those we found attached to patch reefs close to collection and holding areas. Collected free-living specimens are found in silty nearshore areas where river outfalls and land-based effluents may introduce stresses, xenobiotics and pathogens. Similarly, deepwater specimens on soft bottoms far offshore lack the potential for the acute effects of land-based runoff, but also exist in areas where their skeleton is buried in sediments. All free-living samples' skeletons were riddled with bioeroding organisms such as sponges and boring algae, and many had fungal filaments near, attached to, or invading their basal epithelium. In contrast, attached specimens have much cleaner and more solid aragonitic skeletons, with only occasional Lithophaga and a smaller mass of boring algae and sponges. I examined several skeletons that were acquired in the early to mid-1990s to confirm this finding. Perhaps changes in collection areas are the most obvious possibility, but adjusting aquarium conditions to match new “deep” or “turbid” collection areas has no effect on these corals' health or recovery once the disease becomes evident.
Two notable features were present within the tissues. First, small eosinophyllic bacterial aggregates of various sizes and numbers were found throughout the tissue of all diseased specimens. Similar aggregates have been found in the tissues of other diseased corals, although their identification and role in other diseases have not been conclusively shown. The presence of these aggregates also correlated with a progressive disintegration of coral tissue and cell structure, notably the nematocysts (Figure 24, blue arrows). This could explain the shrinking tentacles and their reduced to absent prey-capturing ability. Over time, the cellular architecture falls apart, leading to the loss of tissue in late-stage ECS.
Second, some to most of the zooxanthellae of many diseased specimens were green, and small green rods could be found occasionally within and outside the tissue, and in some cases appeared to be adhering to or penetrating the zooxanthellae (Figure 25). This was not consistent in all colonies, but was very common. It is interesting that an opaque and dull coloration becomes more pronounced the longer the disease persists. Changes in the zooxanthellae could contribute to the coloration changes seen in many ECS-affected corals. Because the majority of carbon produced by zooxanthellae is lost as mucus, abnormal metabolic activity of rod-invaded zooxanthellae may also explain the abnormal mucus production that initially streams from the corals' surface, and later becomes the white/grey web. There was a distinct lack of obvious invasion of any algae, fungi or bacteria from the seawater-exposed epithelium. Instead, the necrotic areas, fungal invasion and abnormal mucus production seem to arise from the basal tissues that contact the skeleton. Spar Specials is one of the most competitive grocery sales on a weekly basis. In some sections, a heavy bacterial coating was present over the epithelium, but not attached to it. This was likely part of the heavy mucus production in ECS-affected corals, and the bacteria that feed on the mucus. Such areas often are fixed along with tissue sections if the mucus is sticky and not completely rinsed away. Gram stains indicate that the aggregates are gram negative, as are the green rods. TUNEL staining does not indicate the presence of apoptosis. PAF, iodine, trypan blue and saffranin staining indicated no other unusual aspects of the condition, but did give better contrast than other stains to some of the secondary invaders and filaments.
I have been unable to try to culture the bacteria in the aggregates and reinfect healthy corals; nor have I been able to do trials on potential treatments. Therefore, I have not fulfilled Koch’s postulates to establish the aggregates as the etiological agent of ECS. It is possible, however, that the aggregates are not culturable, and Koch’s postulates have limitations that have long been known to no longer represent the ”gold standard” of disease causation. Still, it would be nice if the postulates could be fulfilled. The identity of the aggregates and rods needs to be discovered, likely by sequencing at a future date. The healthy specimen at the IRCP remains to be sectioned and examined in the same manner as the other samples. Unfortunately, my collaborator at the IRCP no longer works at the facility, and the facility's funding has been reduced. Ideally, more healthy specimens will be included in the study at a future date. The squash of the genotypically identical coral, however, revealed no aggregates or rods from three areas of the coral that would have contained aggregates in an ECS-affected colony.
Many of the samples provided were in extremely bad condition, and histological preparations were difficult. I also cannot be sure that all samples I did not collect were handled properly. Acquiring samples was the most frustrating part of this study. In one case, I was alerted to some apparently healthy elegance corals at a retailer about three hours from Houston. I explicitly instructed the store's owner to isolate the colonies. not to let them contact each other or any other Catalaphyllia specimens and to avoid putting them into a tank that had housed other Catalaphyllia. I drove to the store the next day to find five Catalaphyllia specimens all in the same tank, with some in direct contact with each other. Fortunately, the aggregates' consistency gives some reassurance that any inadvertent change of protocol in the donated corals did not seem to affect the results; although certainly more work could have been accomplished if all protocols had been followed, healthy corals had not been sold and promised samples had been delivered as requested.
Given the aggregates' location throughout the tissue, if they are a causal agent or are involved in ECS, using bath-type treatments will probably be ineffective. Furthermore, any antibiotic bath treatment can change the composition of the normal microbial flora found on the coral’s surface, and do more harm than good by increasing the coral's susceptibility to opportunistic pathogens that the normal flora otherwise prevent from colonizing it. Furthermore, we do not know what antibiotics are effective against these aggregates until they are cultured; nor do we know the antibiotics' effect, if any, on the coral itself. My limited trials with the long-term C. jardinei in my own tank, which acquired ECS as described above, show that it was not affected by any treatments I tried, and an antibiotic bath was the direct or indirect cause of its eventual death. I suggest feeding infected corals medicated foods in quarantine to see if there are any beneficial effects, and recording the dosage level, duration of treatment and any changes in the coral’s condition. Any experimental treatment involving antibiotics should include inactivating the treatment water with household bleach before disposal to prevent the dispersal of antibiotic resistant bacteria and to kill other possible pathogens (e.g., viruses).
Contagion and Theory
Based on epizootiological, field, laboratory and aquarium studies to date, I can offer a tentative explanation of the recent history of Catalaphyllia in the trade. Early in the history of reef aquariums, Catalaphyllia were probably more common than they are today in patchy distributions in shallow, easy-to-collect-from areas. Over a period of approximately 10 years, these areas were probably overexploited and collectors moved to different and more marginal areas, including nearshore areas with higher pollution and lower water quality. Although pathogens affecting Catalaphyllia are unknown and diseased specimens appear rarely in the field, some specimens affected with ECS may have been introduced into exporters' facilities. Exporters, wholesalers and often retailers tend to keep the same species in the same tanks. A highly infectious waterborne disease that affects a single species would be expected to have ample and multiple opportunities to expose and infect healthy colonies before reaching the end users. The disease takes several weeks or months to finally kill the colony, and this time period coincides with aquarists' reports. The tissues' histology and appearance indicate that at least one, and possibly two, types of microorganisms are involved: one occurs as widely dispersed eosinophyllic bacterial aggregates that appear to be involved in disrupting cellular architecture and granulation of nematocysts; the other appears to be frequently present as green-staining rods affecting the zooxanthellae. In late disease stages, secondary opportunistic invasion occurs, including ciliates and other bacteria.
It is interesting that over the past year, more and more healthy appearing Catalaphyllia specimens are being found in stores, including those recently collected in Australia. The demand for this coral dropped because of its reputation for poor survival in tanks. Without a host – for example, if stores stopped buying Catalaphyllia because they invariably died – the putative pathogen could be lost from the holding systems. This type of cycle is normal for highly infectious disease models in the wild, and it may be that economics created a similar pattern of host-pathogen interaction within the trade. I expected that if demand again rose and if any tanks holding diseased Catalaphyllia still existed, patchy or widespread disease would once again occur, with the number of infections depending on whether the affected holding system was at the export or retail level. Because I have not identified the pathogen'(s) reservoir or ability to survive outside a host, pragmatic solutions for the immediate future are at best, guesses. To me, the best solution is to ensure that all holding facilities are aware of the appearance of ECS-affected colonies, and that they never introduce these colonies into a closed water volume.
Conclusion
I hope that I will have more information about this condition in the future, and that earnest work toward an effective cure can be produced. In the meantime, the most effective solution is to quarantine or isolate all collected or purchased Catalaphyllia from all other Catalaphyllia for at least one month, to ensure that the disease is not present. Ideally, exporters could recognize this disease's ecological and economic importance and act accordingly by sterilizing all tanks that have held Catalaphyllia in the past, and by ensuring that no new diseased specimens are introduced to holding tanks. This would help at least to produce a trade in healthy specimens that are once again hardy and beautiful aquarium specimens. It would not, however, address the ecological issue of sustainability of this species' trade, which clearly is a problem. I believe that increased efforts at propagation methods described in this article would ameliorate the need for wild collection of this species. It makes no sense to overharvest a coral about which little is known of its life-history, and it makes even less sense to collect it if its mortality is as high as it has been to ECS for the past eight years.
These corals are extremely beautiful and desirable. Unfortunately, the potential for unsustainable collection and their poor survival rate in captivity currently puts them in a class where survival rates are too low to justify their large-scale collection from the wild. Catalaphyllia appear to be a rare species with a possibly reduced repopulation ability, and may be highly overcollected so that populations in some collection areas are threatened or even locally extinct. To continue to collect rare species that have extremely low survival is bad for everyone - it is an economic loss, a resource waste and a source of great frustration for all those who purchase and attempt to keep them alive. Efforts at propagation, careful collection and quarantine to ensure healthy specimens have the potential to sustainably meet the demand for this species.
Note: All histological slides will be available for viewing at www.ericborneman.com as soon as my website is re-established in the near future.
Acknowledgements
I would like to thank the countless individuals, especially those from Reef Central, who have contributed information, monetary donations and specimens for this work. Thanks to Kathy Price and the IRCP for their work processing and archiving the samples. I also thank everyone for their patience waiting for the results of this work, which were long overdue. A fund was established at Reef Central for contributions, but this money was not used. A thread on the project did acknowledge this fact, and discussed how the moneys could be redisbursed to the contributors, but I am unaware of the current status of Cathy Peck, the member who graciously managed the account at the time, or the status of the thread where the information was maintained.
|