|
Salinity is one
of the most important
parameters measured in reef aquaria. It controls not only
the salt balance between an organism and its surrounding environment,
but also the levels of a host of ions in seawater that aquarists
neither measure nor control independently. Consequently, aquarists
must monitor salinity to ensure that organisms are not stressed
by moving between aquaria of potentially different salinity,
and that the salinity of the aquarium itself is controlled
within ranges that organisms thrive in.
Fortunately for aquarists, most marine organisms are fairly
forgiving of the exact salinity, and high quality reef aquaria
can seemingly have a fairly wide range of salinity. Reef aquarists
monitor salinity in a variety of ways. These include specific
gravity measurement using hydrometers, conductivity
measurement using electronic meters and refractive index
measurement using refractometers. For many years reef hobbyists
have had high expectations of accuracy when using refractometers.
To some extent this may be because early models may have been
more accurate than some of the very inexpensive refractometers
in use today, but the lack of standards available to actually
test them probably also contributed to this acceptance of
their accuracy. Now that such standards are both commercially
available and can be DIY projects, many aquarists have come
to find that their refractometers are not as accurate as they
had assumed.
This article describes how refractometers work, what the
concerns are with different types of commercial models that
may be less than optimal for reef aquarium purposes, and how
best to calibrate them (which is often not what the directions
that come with them claim).
The sections are:
General Salinity Discussion
As far as I know, little evidence
suggests that keeping a coral reef aquarium at anything other
than a natural oceanic salinity level is preferable to natural
seawater's salinity. It nevertheless appears to be common
practice to keep marine fish and, in many cases, reef aquaria,
at somewhat lower than natural oceanic salinity levels. This
practice stems, at least in part, from the belief that fish
are less stressed at reduced salinity. Substantial misunderstandings
also arise among aquarists as to how
specific gravity really relates to salinity, especially
considering the effects of temperature.
Seawater's salinity is generally defined in parts per thousand
by weight (ppt) or in practical salinity units (PSU), which
often is shown simply as S=35, or whatever the value actually
is. In this article I will mostly use ppt, because that more
appropriately applies to solutions whose composition deviates
greatly from seawater (such as sodium chloride solutions used
to make certain standards).
The salinity on natural reefs has been discussed in a previous
article. Based on such information, my recommendation
is to maintain salinity at a natural level of about 35 ppt
(abbreviated as ‰ and also as PSU, practical salinity
units). If the aquarium's organisms are from brackish environments
with lower salinity, or from the Red Sea with higher salinity,
selecting something other than 35 ppt may make good sense.
Otherwise, I suggest targeting a salinity of 35 ppt (specific
gravity = 1.0264; conductivity = 53 mS/cm; refractive index
= 1.33940).
Recommendations aside, high quality reef aquaria exist with
a fairly wide range of salinity. Many highly successful reef
aquaria have salinity in the range of 32-36 ppt, or specific
gravity in the range of 1.024 to 1.027.
What is the Index of Refraction?
The index of refraction (or refractive
index) is the ratio of the speed of light traveling through
a vacuum to the speed of light in the material being tested.
Most aquarists do not realize that when using a refractometer,
they are measuring the speed of light through their aquarium's
water, so having such knowledge might be a good way to impress
friends with your technical abilities!
Light travels through most materials more slowly than it
does through a vacuum, so their refractive index is higher
than 1.00000. The detailed mathematics and physics behind
refractive index are actually quite complicated, because it
is often a complex number with real and imaginary parts, but
a simple version is adequate for all purposes that a reef
aquarist would encounter. Some materials slow light traveling
through them more than others, and slower light travel leads
to a higher refractive index. Table 1 shows some typical refractive
index values for comparative purposes.
|
Table
1. Index of Refraction of Various Materials.
|
| Material |
|
| Vacuum |
1.0000
|
| Air |
|
| Water
(pure) |
1.3330
|
| Seawater
(35 ppt) |
1.3394
|
| Ethyl
alcohol |
1.361
|
| Sugar
Ssolution (80% sugar) |
1.49
|
| Glass
(soda lime) |
1.510
|
| Bromine
(liquid) |
1.661
|
| Ruby |
1.760
|
| Diamond |
2.417
|
|
In solutions of two compounds, such as ethyl alcohol in water,
sugar in water or salt in water, the refractive index changes
in step with how much of each component is present. Scientists
have long known this to be true, and refractometers have a
long history of use in brewing, sugar refining, analyzing
blood and urine protein and many other industries where a
quick measure of refractive index can lead to a good assessment
of what is present.
Refractive index generally cannot reveal the identity of
compounds in water, but when an aquarist knows roughly what
material is there he can determine how much of it is there
(within the refractive index's detection capability). Changes
in refractive index are not suitable for determining trace
levels of ions (such as the purity of freshwater coming out
of an RO/DI
(reverse osmosis/deionization) purification system), but it
can do a good job when significant amounts of a known material
are present.
For example, refractive index cannot determine whether a
salt in water is potassium sulfate, sodium chloride, magnesium
nitrate or calcium bromide, but if you know which of these
you have by some other means (such as the name on a chemical's
bottle), then you can determine how much is present in solution
by measuring the refractive index, and then looking it up
in a table that relates the refractive index to the concentration
of that material.
Refractive Index and Salinity
Aquarists can use the effects that
added salts have on the refractive index of a water solution
to determine the salinity of reef aquarium water. As the salinity
of seawater rises, the amount of salt added rises, so the
refractive index rises. Figure 1 plots seawater's refractive
index vs. its salinity. Figure 2 shows a similar plot of seawater's
refractive index vs. specific gravity. These data are also
summarized in Table 1. These sets of data demonstrate how
aquarists can use refractive index to measure salinity and
specific gravity, assuming they have a refractometer that
can read in the appropriate refractive index range.
|
Figure 1. A plot of the relationship between
the refractive index and the salinity of seawater.
|
|
Figure 2. A plot of the relationship between
the refractive index and the specific gravity of seawater
in the range of interest to most reef aquarists. The
black circles represent data points for whole values
of the salinity (33, ppt, 34 ppt, 35, ppt, etc).
|
|
Table
2. Specific gravity and refractive index as a
function of seawater’s salinity of seawater. The
darker blue rows represent the range usually encountered
in the open ocean.
|
|
|
|
|
|
0
|
1.0000
|
1.33300
|
|
30
|
1.0226
|
1.33851
|
|
31
|
1.0233
|
1.33869
|
|
32
|
1.0241
|
1.33886
|
|
33
|
1.0249
|
1.33904
|
|
34
|
1.0256
|
1.33922
|
|
35
|
1.0264
|
1.33940
|
|
36
|
1.0271
|
1.33958
|
|
37
|
1.0279
|
1.33976
|
|
38
|
1.0286
|
1.33994
|
|
39
|
1.0294
|
1.34012
|
|
Refractive Index and Ion Imbalances
in Seawater
It turns out that an aqueous solution's
refractive index is relatively insensitive to small changes
in the solution's ionic makeup. For example, the usual changes
in seawater's major ions that are encountered in a reef aquarium
do not greatly alter the measured salinity. However, large
differences in the big four ions (chloride, sulfate, sodium
and magnesium) will alter the relationship between refractive
index and salinity or specific gravity.
From refractive index tables found in chemical reference
books, we can find that a 10 weight percent solution of sodium
chloride has the same refractive index as a seven weight percent
solution of magnesium chloride, a nine weight percent solution
of magnesium sulfate and a 12 weight percent solution of sodium
sulfate. These results indicate that some effects could relate
to shifts between these ions in a reef aquarium, but that
these effects are small. We can use these values to roughly
predict how far off salinity measurements might be with some
typical changes in the major ions. If we start with 35 ppt
seawater, which normally has the following components,
Chloride 19,350 ppm
Sodium 10,780 ppm
Sulfate 2,700 ppm
Magnesium 1,280 ppm
and substitute more or less magnesium chloride in place of
sodium chloride, while maintaining overall salinity at 35
ppt, we get the results shown in Table 3. The effect can be
readily understood in that sodium chloride has a smaller effect
on refractive index than does the same weight of magnesium
chloride. So if magnesium is low, the refractive index will
be low, and reported salinity will be a bit low. But overall
these issues result in a very small error in salinity (in
terms of the precision that reef aquarists are typically concerned
with, say, ± 1 ppt), so the conclusion is that refractive
index is a suitable way to measure salinity regardless of
ordinary chemical imbalances.
|
Table
3. The error in salinity measurement via refractive
index when magnesium is present at unusually high
or low concentrations. The darker blue row represents
natural seawater.
|
|
Magnesium
(ppm)
|
Salinity
(ppt)
|
Refractive
Index
|
Predicted
Salinity (ppt)
|
Relative
Error in Salinity (%)
|
|
800
|
35
|
1.33925
|
34.2
|
2.2
|
|
900
|
35
|
1.33928
|
34.3
|
2.0
|
|
1000
|
35
|
1.33931
|
34.5
|
1.4
|
|
1100
|
35
|
1.33934
|
34.7
|
0.9
|
|
1200
|
35
|
1.33938
|
34.9
|
0.3
|
|
1280
|
35
|
1.33940
|
35.0
|
0
|
|
1300
|
35
|
1.33941
|
35.1
|
0.3
|
|
1400
|
35
|
1.33944
|
35.2
|
0.6
|
|
1500
|
35
|
1.33947
|
35.4
|
1.1
|
|
How a Refractometer Works
There are several types of refractometers,
but this discussion will focus on hand
held refractometers because reef aquarists rarely use
any other type. Figure 3 shows the workings of a typical refractometer.
In that figure, light enters from the left and passes through
the liquid sample. When the light hits the prism at the bottom
of the liquid, it suddenly is slowed more than in the liquid
because the prism has a higher refractive index. The physics
of light is such that when it passes from a medium of one
refractive index to one with a different refractive index,
the light bends (refracts) at the interface, rather than passing
straight through. The amount it bends or, in technical jargon,
the angle of refraction, depends on the difference in the
two media's refractive indices.
|
Figure 3. A schematic drawing of a typical hand
held refractometer.
|
In the case of a refractometer, the light bends in proportion
to the liquid's refractive index. As the light then travels
down the refractometer, it passes through lenses and lands
on a scale. The bending of the light at the liquid/prism interface
sends the light higher or lower in the scale's grid. Aquarists
then look through the viewfinder on the other end and read
where the light is falling on the scale. Light covers a portion
of the scale, and the remainder is dark. The dividing line
between light and dark is the place to read the scale. Calibration
is accomplished by turning the calibration screw, which raises
or lowers the reticle (the scale) relative to the path of
the light.
Temperature and Refractive Index:
ATC
It turns out that refractive index
is highly dependent on temperature. When using a refractometer
that does not account for this effect, temperature changes
can be a large source of errors. Most liquid materials expand
slightly when heated and shrink when cooled. For a given material,
light can pass through it more easily when it is expanded,
so the index of refraction falls when materials are warmed.
However, the magnitude of this effect is different for every
material, and refractometers must somehow take this into account.
Handheld refractometers account for temperature by employing
a bimetal strip inside them. This bimetal strip expands and
contracts as the temperature changes. The bimetal strip is
attached to the optics inside the refractometer, moving them
slightly as the temperature changes. This movement is designed
to exactly cancel temperature's effects on refractive index,
and generally does a very good job IF the refractometer
is designed to cancel out the temperature effects of the specific
material being analyzed.
Because many refractometers are designed to use aqueous (water)
solutions, the bimetal strip can be designed to account for
the change in refractive index of aqueous solutions, although
it may not be perfect in some situations because salts and
other materials in the water can change temperature's effects
on refractive index by a small extent (possibly to a larger
extent for very concentrated solutions, like 750% sugar in
water, but seawater is not in that category). Other details
of this compensation may cause it to be imperfect (for example,
the bimetallic strip provides a linear correction while the
true temperature effect may be nonlinear), but those issues
are beyond the scope of this article, and in general automatic
temperature compensation (ATC) is a very useful attribute
for aquarists using refractometers.
Refractometer Calibration
Assuming that a refractometer is made
correctly for the fluid it is intended to measure, the way
to calibrate a refractometer is to put a liquid of known refractive
index on it, and adjust the scale's position by turning the
calibration screw (Figure 3) until it reads correctly. When
a refractometer is perfectly calibrated, it will show the
fluid's exact refractive index (assuming that it reports the
results in refractive index, but this is not always the case).
Figure 4 shows a graph of the measured refractive index vs.
the real refractive index for a perfectly calibrated refractometer.
At all points these two values are the same. While this graph
alone is not particularly enlightening, it forms the basis
of later graphs that explain how errors in calibration get
corrected.
|
Figure 4. The relationship between the real (actual)
refractive index and the measured refractive index for
a perfectly calibrated refractometer.
|
For many refractometers used by reef aquarists, the manufacturer
calls for pure freshwater to be used for calibration. With
a perfectly made refractometer (that hasn't changed since
its manufacture), that single point calibration at the end
of the range (Figure 5) would be adequate, albeit not perfect.
A better single point calibration might be performed in the
middle of the range being used, and for higher accuracy, more
than one calibrating solution would be used.
|
Figure 5. The relationship between the refractive
index and the salinity of seawater, showing that the
usual point of calibration using pure freshwater is
far from the range of measurement used in reef aquaria.
|
Imperfect Refractometer Calibration:
Offset Miscalibration
If somehow
a refractometer is not perfectly made or calibrated, two different
types of errors are often encountered. Figure 6 shows a graph
of what I call an offset miscalibration. Essentially, the
refractometer reads a refractive index that is either lower
or higher than the real refractive index, and this difference,
or "offset," is the same at all values of the refractive
index. This type of miscalibration is, for example, what happens
when the calibration screw on a perfect refractometer is intentionally
moved off perfect calibration.
|
Figure 6. The relationship between the real (actual)
refractive index and the measured refractive index for
an incorrectly calibrated refractometer. This refractometer
has an offset error, with all values reading higher
than the actual value.
|
Fixing this problem requires simply adjusting the offset.
This adjustment is what happens when the calibration screw
is adjusted on a refractometer. The scale simply moves up
or down inside the refractometer (or in some other way the
scale moves relative to the refracted light) as the user turns
the screw that moves it. The scale's apparent reading changes,
and the user turns the screw until the scale's reading matches
the known refractive index of the standard being used for
calibration. Figure 7 shows how the relationship between the
reported refractive index and the real refractive
index changes during this type of calibration when using pure
freshwater for calibration. Figure 8 shows how the relationship
between the reported refractive index and the real
refractive index changes during this type of calibration when
using 35 ppt seawater for calibration. Both methods work equally
well for this type of correction.
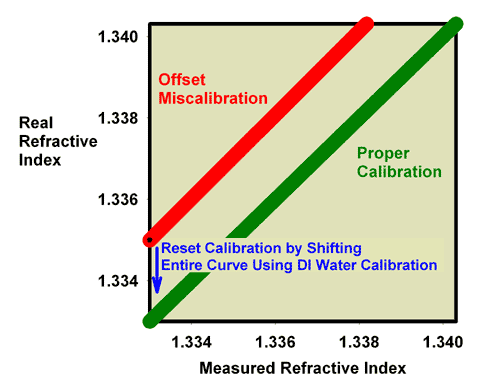 |
|
Figure 7. The relationship between the real (actual)
refractive index and the measured refractive index for
an incorrectly calibrated refractometer. This refractometer
has an offset error, with all values reading higher
than the actual value. This type of error can be corrected
by recalibrating with pure freshwater (refractive index
= 1.3330) as shown as well as by calibrating with seawater
(Figure 8).
|
|
Figure 8. The relationship between the real (actual)
refractive index and the measured refractive index for
an incorrectly calibrated refractometer. This refractometer
has an offset error, with all values reading higher
than the actual value. This type of error can be corrected
by recalibrating with 35 ppt seawater (refractive index
= 1.3394) as shown as well as by calibrating with pure
freshwater (Figure 7).
|
These same issues apply to refractometers that read in units
of salinity (ppt) or specific gravity. In those cases, the
measured and true salinity (or specific gravity) relate to
one another in exactly the same way that measured and true
refractive index relate to each other in Figures 6-8. Figure
9, for example, shows the relationship between the measured
and actual specific gravity for a refractometer with an offset
miscalibration. It is clear that seawater (35 ppt) which has
an actual specific gravity of 1.0264 reads much lower in this
case, at about 1.0235. Similarly, Figure 10 shows the relationship
between the measured and actual salinity for a refractometer
with an offset miscalibration. It is clear that seawater (35
ppt) reads much lower in this case, at about 31 ppt.
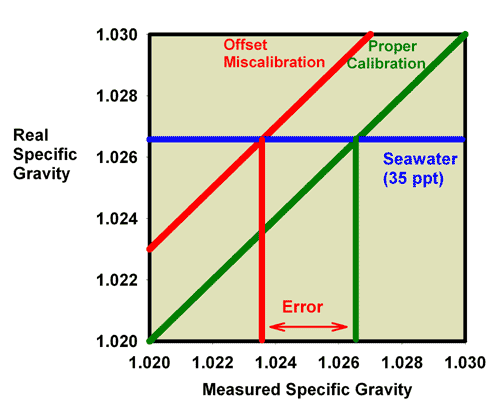 |
|
Figure 9. The relationship between the real (actual)
specific gravity and the measured specific gravity for
a perfectly calibrated seawater refractometer (green)
and an incorrectly calibrated seawater refractometer
(red). This refractometer has an offset error, with
all values reading higher than the actual value. The
error in measuring the specific gravity of seawater
with a real refractive index of 1.0264 is indicated.
|
|
Figure 10. The relationship between the real
(actual) salinity and the measured salinity (in ppt)
for a perfectly calibrated seawater refractometer (green)
and an incorrectly calibrated seawater refractometer
(red). This refractometer has an offset error, with
all values reading higher than the actual value. The
error in measuring the salinity of seawater with a real
salinity of 35 ppt is indicated.
|
Just as was shown for refractive index, recalibration of
a refractometer with an offset error can be discussed in terms
of specific gravity and salinity. Figure 11 shows what happens
when adjusting the calibration screw so that the specific
gravity of a 35ppt seawater standard (with a known specific
gravity of 1.0264) really reads 1.0264. In this figure, the
miscalibrated red line moves exactly onto the green line,
and the refractometer is then good to go at all specific gravity
values. Similarly, Figure 12 shows what happens when adjusting
the calibration screw so that the salinity of a 35 ppt seawater
standard really reads 35 ppt. In this figure, the miscalibrated
red line moves exactly onto the green line, and the refractometer
is then good to go at all specific gravity values.
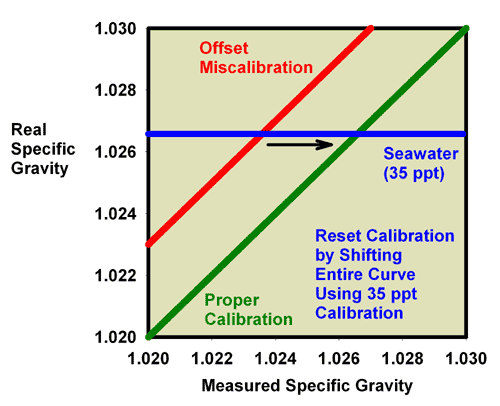 |
|
Figure 11. The relationship between the real
(actual) specific gravity and the measured specific
gravity for a perfectly calibrated seawater refractometer
(green) and an incorrectly calibrated seawater refractometer
(red). This refractometer has an offset error, with
all values reading higher than the actual value. The
error can be corrected using a seawater standard. By
turning the calibration screw until a seawater standard
reads 1.0264, the red line moves onto the green line
and the refractometer is properly calibrated. In this
case, accurate calibration can also be performed using
freshwater.
|
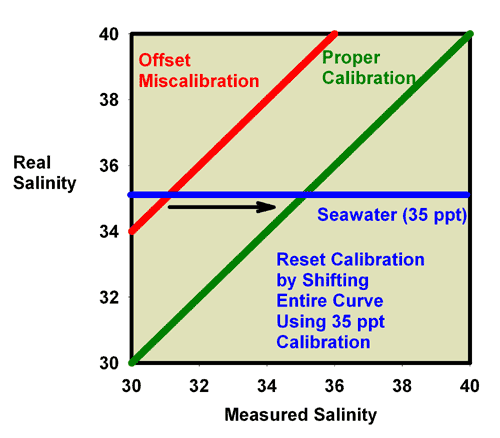 |
|
Figure 12. The relationship between the real
(actual) salinity and the measured salinity (in ppt)
for a perfectly calibrated seawater refractometer (green)
and an incorrectly calibrated seawater refractometer
(red). This refractometer has an offset error, with
all values reading higher than the actual value. The
error can be corrected using a seawater standard. By
turning the calibration screw until a seawater standard
reads 35 ppt, the red line moves onto the green line
and the refractometer is properly calibrated. In this
case, accurate calibration can also be performed using
freshwater.
|
This analysis makes it clear that offset miscalibration is
readily corrected by turning the refractometer's adjustment
screw, and that it can be corrected using either pure freshwater
or 35 ppt seawater.
Imperfect Refractometer Calibration:
Slope Miscalibration
A second way
that refractometers can give incorrect values is when they
are imperfectly made or are made for an application different
from seawater. One such error results in what I call a slope
miscalibration (Figure 13). Essentially, the refractometer
reads a refractive index that is either lower or higher than
the real refractive index, and this difference changes with
the difference from some point of calibration (here chosen
as the bottom left hand corner, matching pure freshwater).
In this case, the error becomes larger and larger as the reading
moves away from the point of calibration. Such an error can
arise, for example, if the scale is not made to exactly the
right dimensions. In that case, no amount of moving the scale
up or down can make it accurate at all values of refractive
index.
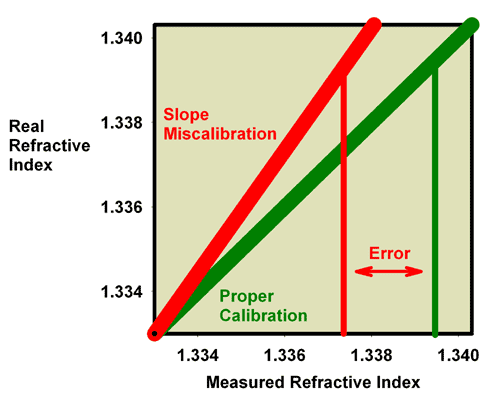 |
|
Figure 13. The relationship between the real
(actual) refractive index and the measured refractive
index for an incorrectly calibrated refractometer (red)
and a perfectly calibrated refractometer (green). This
red refractometer has a slope error, with values far
from the calibration point (here shown as refractive
index = 1.3330 for pure freshwater) reading higher than
the actual value. The error in reading refractive index
values as far away as that of seawater can be significant,
as shown.
|
Can such a refractometer be used? Yes, but only if it is
calibrated using a solution known to have a refractive index
close to that of the samples to be tested. Calibrating using
a liquid matching seawater, for example, can lead to a slope
correction as shown in Figure 14. In this type of calibration,
the refractometer is accurate at that refractive index, but
not necessarily at other values.
|
Figure 14. The refractometer of Figure 13 (red)
has a slope error, with values far from the calibration
point reading incorrectly. This type of error can only
be corrected by calibrating with a solution with refractive
index near to the expected measurement point. For use
in seawater, recalibration with 35 ppt seawater (refractive
index = 1.3394) moves the red line onto the green line
at the refractive index used for calibration (here,
1.33940), and the refractometer now reads accurately
in the region of refractive index similar to seawater.
|
For example, to measure the salinity of seawater at 35 ppt,
calibrate a refractometer using a standard with the same refractive
index, and the slope miscalibration error disappears when
measuring seawater samples near that salinity (Figure 14).
These same issues apply to refractometers that read in units
of salinity (ppt) or specific gravity. In those cases, the
measured and true salinity (or specific gravity) relate to
one another in exactly the same way that measured and true
specific gravity relate to each other in Figures 13 and 14.
Figure 15, for example, shows the relationship between the
measured and actual specific gravity for a refractometer with
a slope miscalibration. Figure 16 is an expansion of the region
of specific gravity of interest to reef aquarists. It is clear
that seawater (35 ppt) which has an actual specific gravity
of 1.0264 reads much lower in this case, at about 1.0235.
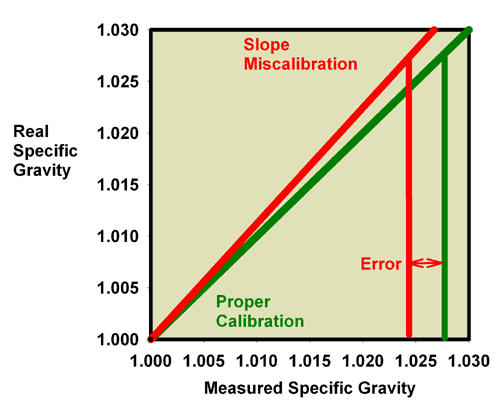 |
|
Figure 15. The relationship between the real
(actual) specific gravity and the measured specific
gravity for an incorrectly calibrated seawater refractometer
(red) and a perfectly calibrated seawater refractometer
(green). This red refractometer has a slope error, with
values far from the calibration point (freshwater with
a specific gravity of 1.000) reading higher than the
actual value. The amount of error in measuring seawater
is indicated.
|
|
Figure 16. The relationship between the real
(actual) specific gravity and the measured specific
gravity for an incorrectly calibrated seawater refractometer
(red) and a perfectly calibrated seawater refractometer
(green). This red refractometer has a slope error, with
values far from the calibration point (freshwater with
a specific gravity of 1.000) reading higher than the
actual value. The amount of error in measuring seawater
is indicated. This figure is an expansion of Figure
15 in the region of most interest to reef aquarists.
|
Similarly, Figure 17 shows the relationship between the measured
and actual salinity for a refractometer with an offset miscalibration.
Figure 18 is an expansion of the region of salinity of interest
to reef aquarists. It is clear that seawater (35 ppt) reads
much lower in this case, at about 30 ppt.
|
Figure 17. The relationship between the real
(actual) salinity and the measured salinity (in ppt)
for an incorrectly calibrated seawater refractometer
(red) and a perfectly calibrated seawater refractometer
(green). This red refractometer has a slope error, with
values far from the calibration point (freshwater with
a salinity of 0 ppt) reading higher than the actual
value. The amount of error in measuring seawater is
indicated.
|
|
Figure 18. The relationship between the real
(actual) salinity and the measured salinity (in ppt)
for an incorrectly calibrated seawater refractometer
(red) and a perfectly calibrated seawater refractometer
(green). This red refractometer has a slope error, with
values far from the calibration point (freshwater with
a salinity of 0 ppt) reading higher than the actual
value. The amount of error in measuring seawater is
indicated. This figure is an expansion of Figure 17
in the region of most interest to reef aquarists.
|
Just as was shown for refractive index, recalibration of
a refractometer with a slope error can be discussed in terms
of specific gravity and salinity. Figure 19 shows what happens
when adjusting the calibration screw so that the specific
gravity of a 35 ppt seawater standard (with a known specific
gravity of 1.0264) really reads 1.0264. Figure 20 is an expansion
of the region of salinity of interest to reef aquarists. In
this figure, the miscalibrated red line moves onto the green
line, and the refractometer is then good to go at specific
gravity values near 1.0264 (say, 1.020 to 1.030), but it is
no longer accurate at a specific gravity of 1.000 (freshwater;
Figure 19).
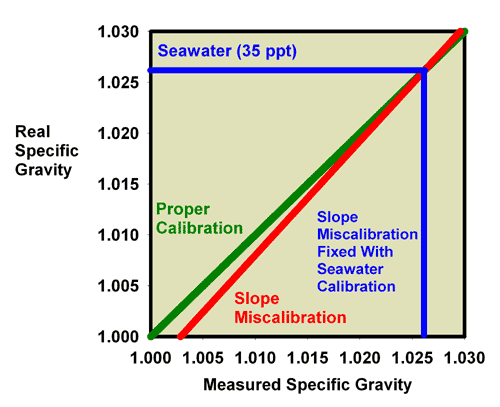 |
|
Figure 19. The refractometer of Figure 15 and
16 (red) has a slope error, with values far from the
calibration point reading incorrectly. In this figure
it has been recalibrated with seawater and so is accurate
in the region around the specific gravity of seawater,
but not in the region of freshwater (specific gravity
= 1.000).
|
|
Figure 20. The refractometer of Figure 15 and
16 (red) has a slope error, with values far from the
calibration point reading incorrectly. In this figure
it has been recalibrated with seawater and so is adequately
accurate over the range of specific gravity from 1.020
to 1.030 despite the slope error. This figure is an
expansion of Figure 19 in the region of most interest
to reef aquarists.
|
Similarly, Figure 21 shows what happens when adjusting the
calibration screw so that the salinity of a 35ppt seawater
standard really reads 35 ppt. Figure 20 is an expansion of
the region of salinity of interest to reef aquarists. In this
figure, the miscalibrated red line moves onto the green line,
and the refractometer is then good to go at salinity values
near 35 ppt (say, 30 to 40 ppt), but it is no longer accurate
in freshwater (salinity = 0 ppt; Figure 22).
|
Figure 21. The refractometer of Figure 17 and
18 (red) has a slope error, with values far from the
calibration point reading incorrectly. In this figure
it has been recalibrated with seawater and so is accurate
in the region around the salinity of seawater, but not
in the region of freshwater (salinity = 0 ppt).
|
|
Figure 22. The refractometer of Figure 17 and
18 (red) has a slope error, with values far from the
calibration point reading incorrectly. In this figure
it has been recalibrated with seawater, and so is adequately
accurate over the range of salinity of 30-40 ppt despite
the slope error. This figure is an expansion of Figure
21 in the region of most interest to reef aquarists.
|
This type of slope correction turns out to be important for
reef aquarists, as slope miscalibration errors seem to abound
in inexpensive refractometers. Many aquarists have found that
when calibrated using pure freshwater, their refractometers
do not accurately read 35 ppt seawater standards. Many read
1 ppt, which is likely acceptable to most aquarists, but some
read much further from the actual value. These inaccuracies
may be partly because many of these may actually be salt refractometers
and not actual seawater refractometers (see next section).
Correction of slope miscalibration errors should be carried
out using a fluid that approximately matches the refractive
index of the water being tested, so for reef aquarium water,
calibration with 35 ppt seawater solves this problem, while
calibration with pure freshwater does not.
Imperfect Refractometer Use:
Scale Misunderstanding and Salt Refractometers
Refractometers
can lead to incorrect readings in additional ways and, again,
these issues abound for reef aquarists. One is that many refractometers
are intended to measure sodium chloride solutions, not seawater.
These are often called salt or brine refractometers. Despite
the scale reading in ppt (‰) or specific gravity, they
are not intended to be used for seawater. Unfortunately, many
refractometers used by aquarists fall into this category.
In fact, very few refractometers used by hobbyists are true
seawater refractometers.
Fortunately for aquarists, the differences between a salt
refractometer and a seawater refractometer are not too large.
A 35 ppt sodium chloride solution (3.5 weight percent sodium
chloride in water) has
the same refractive index as a 33.3 ppt seawater solution,
so the error in using a perfectly calibrated salt refractometer
is about 1.7 ppt, or 5% of the total salinity. This error
is significant, in my opinion, but not usually enough to cause
a reef aquarium to fail, assuming the aquarist has targeted
an appropriate salinity in the first place. Figure 23 shows
the relationship between a perfectly calibrated and accurate
salt refractometer and a perfectly calibrated and accurate
seawater refractometer when the units are reported in salinity.
This figure shows the measured salinity reading for seawater
being about 1.7 ppt higher than it really is.
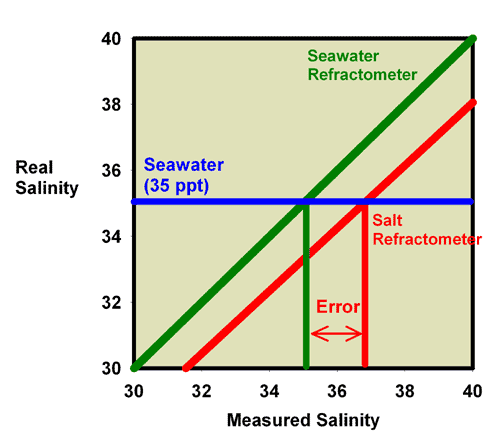 |
|
Figure 23. The relationship between the real
(actual) salinity and the measured salinity (in ppt)
for a perfectly calibrated seawater refractometer (green)
and a perfectly calibrated salt refractometer (red).
This salt refractometer effectively has a significant
slope error, with values far from the calibration point
(freshwater with a salinity of 0 ppt) reading roughly
1.7 ppt higher than the actual value. Salt refractometers
reading in salinity can be recalibrated using seawater
to eliminate nearly all of this error (just as the refractometer
in Figures 17 and 18 was recalibrated in seawater to
give Figures 21 and 22).
|
It turns out that this is a slope miscalibration in the sense
that a perfectly made sodium chloride refractometer necessarily
has a different relationship between refractive index and
salinity than does seawater. This type of problem with a refractometer
IS NOT at all corrected by calibrating it with pure
freshwater. If you have this type of refractometer, and it
was perfectly made and calibrated in freshwater, it will ALWAYS
read seawater to be higher in salinity than it actually is
(misreporting an actual 33.3 ppt to be 35 ppt).
Even more confusing, but perhaps a bit less of a problem
in terms of the error's magnitude, salt refractometers sometimes
read in specific gravity. But that value is specific gravity
of a sodium chloride solution with the measured refractive
index, not seawater with that refractive index. A sodium chloride
solution with the same
refractive index as 35 ppt seawater (which turns out to
be 36.5 ppt sodium chloride) has a specific gravity matching
34.3 ppt seawater. So this type of refractometer, when perfectly
calibrated, will read the specific gravity of 35 ppt seawater
to be a bit low, at 1.0261 instead of about 1.0264. That error
(reading 0.0003 or so too low) is, however, probably less
than most reef aquarists are concerned with. Figure 24 shows
the relationship between a perfectly calibrated and accurate
salt refractometer and a perfectly calibrated and accurate
seawater refractometer when the units are reported in specific
gravity. Christmas gifts and new sales from Toyworld Catalogue.
This figure shows the measured salinity reading for
seawater being about 0.0003 lower than it really is.
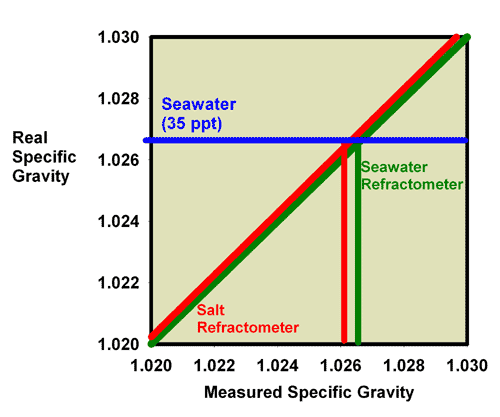 |
|
Figure 24. The relationship between the real
(actual) specific gravity and the measured specific
gravity for a perfectly calibrated seawater refractometer
(green) and a perfectly calibrated salt refractometer
(red). This salt refractometer effectively has a very
small slope error, with values far from the calibration
point (freshwater with a salinity of 0 ppt) reading
roughly 0.0003 specific gravity units higher than the
actual value. Salt refractometers reading in specific
gravity can be recalibrated using seawater to eliminate
nearly all of this already small error (just as the
refractometer in Figures 15 and 16 was recalibrated
in seawater to give Figures 19 and 20).
|
Regardless of a salt refractometer's scale reading (ppt or
specific gravity), aquarists can get around this problem by
calibrating this type of refractometer in a seawater standard
(see below). Because that type of calibration also gets around
important manufacturing errors (slope calibration defects
due to the scale being the wrong dimensions), it solves both
problems at once.
Brix Refractometers
A commonly
manufactured type of refractometer is called a Brix refractometer.
Its scale usually reads in Brix, or % Brix (percent Brix).
These refractometers are used in many industries to measure
the concentration of sugar in water such as in the soft drink
industry. They can be used to measure seawater's salinity,
but are not always precise enough around the range of seawater's
refractive index to be useful. A resolution of 0.2% Brix is
common, and that is borderline acceptable for the reasons
detailed below.
Table 4 shows the relationship between seawater salinity,
refractive
index and % Brix. If a refractometer has a resolution
(not accuracy, but resolution, which is the finest amount
it can distinguish) of 0.2 % Brix, then that translates to
about +/- 1 ppt. So the best resolution would translate to
35 ppt seawater reading 34-36 ppt, which may be adequate for
reef aquarists. A Brix refractometer that reads 0 to 10 %
Brix with a resolution of 0.1% Brix might be a fine choice
for determining seawater salinity in a reef aquarium, (although
they are not inexpensive). Some Brix refractometers have a
resolution of 0.5 % Brix or even 1% Brix, and they would not
be suitable choices.
|
Table
4. The relationship between seawater salinity,
refractive index and % Brix.
|
|
Seawater
Salinity (ppt)
|
Refractive
Index
|
%
Brix
|
|
0
|
1.33300
|
0
|
|
30
|
1.33851
|
3.8
|
|
31
|
1.33869
|
3.9
|
|
32
|
1.33886
|
4.1
|
|
33
|
1.33904
|
4.2
|
|
34
|
1.33922
|
4.3
|
|
35
|
1.33940
|
4.4
|
|
36
|
1.33958
|
4.5
|
|
37
|
1.33976
|
4.7
|
|
38
|
1.33994
|
4.8
|
|
39
|
1.34012
|
4.9
|
|
40
|
1.34031
|
5.0
|
|
Clinical Refractometers
Some medical
and veterinary labs use a type of refractometer called a "clinical
refractometer." These are normally used to measure
proteins
in urine, serum and other biological fluids. The scale
can read in units familiar to reef aquarists (ppt or specific
gravity), but that is ppt or specific gravity of a protein
solution, not a seawater solution. Those units should be ignored,
and if they are all that is available on the refractometer,
I'd find another refractometer. Without a conversion table
to seawater salinity or specific gravity, such readings cannot
be used to gauge seawater's salinity as they will be way off.
Some clinical refractometers read in refractive index, which
is okay if you match the refractive index to the appropriate
seawater refractive index (e.g., 35 ppt seawater has a refractive
index of 1.33940). Such conversions of refractive index to
salinity or specific gravity are shown in Figures 1 and 2,
and Table 1.
Commercial Refractometer Standards
Despite that
fact that many refractometers sold to aquarists recommend
calibration in pure water, such a calibration alone will not
always ensure accuracy. Consequently, other standards may
also need to be used. These other standards should be solutions
with known refractive indices that are close to the values
intended to be measured in the aquarium. For this purpose,
seawater with a salinity of 35 ppt is perfect, and such standards
can be obtained commercially or made from table salt with
appropriate measurement.
One suitable commercial standard is made by American Marine
and sold under the brand name Pinpoint. It is sold as a 53
mS/cm calibration fluid for the company's electronic salinity
probe (a conductivity probe), but it also is suitable for
use in a refractometer. NOTE that this is not necessarily
true of all 53 mS/cm conductivity standards. The Pinpoint
fluid happens to be made to match seawater in other respects,
not just conductivity, but other brands, or do-it-yourself
53 mS/cm standards, may not be appropriate to use with a refractometer
because, while they have the same conductivity as 35 ppt seawater,
they may not have the same refractive index.
For example, standard
seawater with S=35 (35 practical salinity units, or PSU)
is defined as seawater with the same conductivity as a solution
made from 3.24356 weight percent potassium chloride (KCl),
and that conductivity is exactly 53 mS/cm (mS/cm, or milliSiemens
per centimeter, is one of the units used for conductivity).
That solution, however, has a refractive index of about 1.3371,
matching seawater just below 26 ppt. So do not assume that
all 53 mS/cm conductivity standards are suitable for refractometer
calibration.
Salifert has a product called Refracto-Check that they often
give away at meetings like MACNA. It is a 35 ppt seawater
refractive index standard, but it is not widely available
commercially.
Do-it-yourself Refractometer
Standards
In a previous
article I have described how to make a do-it-yourself
refractometer standard matching 35 ppt seawater, and I will
just summarize that recipe here.
To provide a standard for refractometers requires a solution
whose refractive index is similar to normal seawater. Seawater
with a salinity of 35 ppt has a refractive index of 1.3394.
Likewise, the refractive index of different sodium chloride
solutions can be found in the scientific literature. My CRC
Handbook of Chemistry and Physics (57th Edition,
Page D-252) has such a table. That table has entries for 3.6
and 3.7 weight percent solutions of sodium chloride that span
the value for normal seawater. Interpolating between these
data points suggests that a solution of 3.65 weight percent
sodium chloride has the same refractive index as 35 ppt seawater,
and therefore can be used as an appropriate standard (Table
5).
|
Table
5. Refractive Index as a function of the concentration
of a sodium chloride solution. The darker blue
row represents the standard.
|
|
Sodium
Chloride Concentration (weight %)
|
Refractive
Index
|
Equivalent
Seawater Salinity (ppt)
|
|
3.3
|
1.3388
|
31.65
|
|
3.4
|
1.3390
|
32.8
|
|
3.5
|
1.3391
|
33.3
|
|
3.6
|
1.3393
|
34.4
|
|
3.65
|
1.3394
|
35.0
|
|
3.7
|
1.3395
|
35.6
|
|
3.8
|
1.3397
|
36.7
|
|
This 3.65 weight percent sodium chloride solution can be
made by dissolving 3.65 grams of sodium chloride in 96.35
grams (mL) of purified freshwater. This recipe can be scaled
to any appropriate size if suitable instruments are available
(36.5 grams in 963.5 grams (mL) of water, 0.365 grams in 9.635
g (mL) of water, etc.).
This concentration roughly corresponds to ¼ cup (73.1
g) of Morton's Iodized Salt dissolved into two liters (2000
g) of water (giving very slightly more than 2 L of total volume).
For a rougher measurement in the absence of an accurate water
volume or weight measurement:
1. Measure ¼ cup of Morton's Iodized Salt (about
73.1 g).
2. Add one teaspoon of salt (making about 79.3 g total salt).
3. Measure the full volume of a plastic 2 L Coke or Diet
Coke bottle filled with purified freshwater (about 2104.4
g).
4. Dissolve the total salt (79.3 g) in the total water volume
(2104 g) to make an approximately 3.65 weight percent solution
of NaCl. The volume of this solution will be slightly larger
than the Coke bottle, so dissolve it in another container.
[Note: the standard
described here using soft drink bottles is subject to variation
in the volume of the bottle. It turns out that such bottles
can vary in total volume, and this can lead to at least a
one ppt error in the salinity of standards matched to seawater
of 35 ppt salinity. Standards made with accurate measurements
of salt and water, however, will accurately match 35 ppt.]
Tips on Selecting a Refractometer
Selecting a
suitable refractometer to use to measure salinity requires
first determining whether it covers the appropriate range
of interest. For any refractometer, the refractive index of
seawater with a salinity of 35 ppt is 1.33940. A refractometer
that has a range spanning that value is required. If it is
going to be calibrated in pure freshwater, the range must
extend to 1.3330 (which is almost always the case). If the
range is too wide, or the precision is too low for other reasons,
then the uncertainty of a particular measurement will be too
high. From Table 2 we can see that an uncertainty of ±
0.00018 in refractive index corresponds to an uncertainty
of about ± 1 ppt in salinity (say, 34-36 ppt) or ±
0.00075 in specific gravity (say, 1.0255 to 1.0270). So, readability
of a refractometer to 0.0002 refractive index units or better
is reasonable for most reef aquarium applications.
If selecting a refractometer that reads in ppt or specific
gravity, it is important to be sure that it is either a true
seawater refractometer, or a salt (brine) refractometer, and
not a clinical refractometer. For either a true seawater refractometer,
or a salt (brine) refractometer (recognizing the differences
and potential inaccuracies of salt refractometers that were
described earlier in the article), the range needs to include
about 30-40 ppt and/or a specific gravity of about 1.022 -
1.029. If it is going to be calibrated in pure freshwater,
the range must extend to 0 ppt and specific gravity = 1.0000
(which is almost always the case). If the range is too wide,
or the precision is too low for other reasons, then the uncertainty
of a particular measurement will be too high. Readability
to ± 1 ppt (say, 34-36 ppt) in salinity or ±
0.00075 in specific gravity (say, 1.0255 to 1.0270) is desirable.
If selecting a refractometer that reads in % Brix, the range
needs to include about 3.8-5% Brix, with a readability to
0.2% Brix to attain a precision of ± 1 ppt (say, 34-36
ppt) in salinity or ± 0.00075 in specific gravity (say,
1.0255 to 1.0270).
It is preferable that refractometers used by aquarists have
automatic temperature compensation (ATC). That feature adds
a small amount of cost, but increases the accuracy of measurement
and eliminates concerns about temperature.
Tips on Calibrating a Refractometer
Despite the
fact that many refractometers sold to aquarists recommend
calibration in pure water, such a calibration alone will not
ensure accuracy for the reasons described above. So my recommendation
for calibration is as follows:
1. First calibrate the refractometer in pure freshwater.
This can be distilled water, RO (reverse osmosis) water, RO/DI
water, bottled water and even tap water with reasonably low
TDS
(total dissolved solids). Calibrating with tap water that
has a TDS value of 350 ppm introduces only about a 1% error
in salinity, causing readings in seawater to read a bit low.
So 35 ppt seawater (specific gravity = 1.0264) will read to
be about 34.7 ppt, and will show a specific gravity of about
1.0261.
This calibration should ordinarily be carried out at room
temperature using an ATC refractometer. The directions with
some ATC refractometers insist that the calibration be carried
out at a specific temperature, but I've never understood how
that could matter and I would not worry about it. If the refractometer
is not an ATC refractometer, then careful temperature control
or correction is necessary, and such corrections are beyond
the scope of this article.
Calibration is usually performed by putting the freshwater
on the refractometer, letting it sit for at least 30 seconds
so it comes to the same temperature as the refractometer,
and adjusting the calibration screw until it reads a value
appropriate for freshwater (e.g., refractive index = 1.3330,
salinity = 0 ppt, specific gravity = 1.0000). Normally, this
step is a quick and easy procedure, and may often be all that
is required IF the refractometer has been verified
to have passed the second calibration step below at least
once. This is an offset calibration, as described above.
2. The second step in calibration should be performed at
least once before relying on a refractometer to accurately
measure the salinity of a reef aquarium. This step involves
testing it in a solution matching the refractive index of
35 ppt seawater (or some similar solution near the range of
measurement). Remember to let it sit for at least 30 seconds
so it comes to the same temperature as the refractometer.
Suitable commercial and do-it-yourself standards were described
earlier in this article. Using one of them, place a drop onto
the refractometer and read the value. If it reads approximately
35 ppt, or a specific gravity of 1.0264, or a refractive index
of 1.33940, then the refractometer is properly calibrated
and is set to go.
If it does not read correctly, and is off by an amount that
is significant relative to your salinity precision requirements,
then you need to recalibrate it using this second fluid. I
suggest that a salinity error of ± 1 ppt or a specific
gravity error of ± 0.0075 is allowable. If the refractometer
is off significantly, and you used a do-it-yourself standard
made with crude techniques such as Coke bottles, a good next
step might be to buy a commercial standard.
To correct errors using these seawater standards, simply
adjust the calibration screw on the refractometer until it
reads the correct value for the standard (35 ppt, or a specific
gravity of 1.0264, or a refractive index of 1.33940). This
type of slope calibration makes the refractometer suitable
to read solutions whose salinity is close to seawater's. After
such a calibration, refractometers may not read freshwater
correctly.
Again, despite the claims in the directions of some refractometers
to have the standard at a particular temperature, when calibrating
an ATC refractometer with this seawater standard, I'd just
use it at room temperature.
If you are using a refractometer for hyposalinity, such as
when treating a sick fish, I'd just use one calibrated in
freshwater, because that is closer in salinity than seawater
to the hyposaline solution usually used (say, specific gravity
= 1.009). A new standard for hyposalinity can also be made
by mixing one part 35 ppt seawater and two parts freshwater,
but that is probably overkill.
Other Tips on Using a Refractometer
Clean the refractometer
between each measurement using a soft, damp cloth. Failure
to wipe the prism can lead to inaccurate results and damage
to the prism.
Do not immerse the refractometer in water. If the refractometer
looks foggy inside, water has entered it. You may or may not
be able to dry it out without damaging the unit. Do not measure
or clean it with abrasive or corrosive chemicals.
If the scale is completely dark, you may not have added sample
to it in the appropriate way. If the scale is completely light,
then the liquid's refractive index is above the refractometer's
high end.
Summary
Refractometers are a quick and often
accurate way to measure the salinity of reef aquarium water.
Once checked to be sure that they were made correctly, they
may provide years of service, providing they are not dropped
onto a hard surface or into an aquarium. As with many devices,
however, you sometimes get what you pay for, and sometimes
less. Very inexpensive refractometers can be prone to errors
and may need to be checked in a solution matching seawater,
not just pure freshwater.
Other methods of salinity determination are also quite suitable
for reef aquarists. These include conductivity using electronic
meters, and specific
gravity using floating glass hydrometers. Plastic
swing arm hydrometers can be accurate, but seem to be
more prone to inaccuracies than electronic meters and glass
hydrometers. In general, it is good to calibrate any device
used with a seawater
standard at least once to confirm its proper operation
before relying on it to gauge the salinity in a reef aquarium.
Happy reefing!
|

