|
Introduction
Reef organisms take
up and use nutrients from their environment in order to support
their metabolism, to grow and to reproduce. Even though the
concentrations of dissolved inorganic nitrogen and phosphorus
(DIN and DIP) are very low in the water washing over coral
reefs, they are still sufficient to maintain very high rates
of primary productivity. This high rate of productivity necessitates
a high availability of essential nutrients such as carbon,
nitrogen and phosphorus. One of the sources of these materials
for coral reefs, as examined last month, is the land located
near most coral reefs. Not all reefs are located near land,
however, so land cannot represent the only possible source
of essential nutrients for reef ecosystems. Indeed, it does
not. Coral reefs are connected not only to nearby land, but
also to the sky above them. Reef organisms actively take up
nutrients that originate in the overlying atmosphere and some
amount of nutrients from reefs is lost to this pool as well.
It's Raining… Nitrogen?
Before the advent
of the Haber-Bosch industrial nitrogen fixation process, the
vast majority of the combined nitrogen available to any ecosystem
was first made available due to the biological fixation of
atmospheric nitrogen by N-fixers. Actinomycetes and rhizobium
bacteria, along with lichens (symbiotic cyanobacterial and
fungal associations), were, and still are, the primary N-fixers
on land, while cyanobacteria are much more important in aquatic
ecosystems. However, life evolved before the biological ability
to fix atmospheric nitrogen. The combined nitrogen available
to early life forms was fixed primarily by lightning. As lightning
travels through air it produces nitric oxide (NO) and nitrogen
dioxide (NO2) (collectively known as NOx-rhymes
with box) from molecular nitrogen and oxygen. Much of the
nitric oxide produced by lightning can be oxidized to nitrogen
dioxide by ozone, or even molecular oxygen, to produce nitrogen
dioxide (Hill et al, 1980). The NO2 can
then react with water molecules to form nitric acid. The nitrate
ion resulting from the formation of nitric acid is then deposited
dry as an aerosol or wet in rainwater. This atmospheric source
of nitrogen, which was of major importance to the first life
forms, is still formed today. While it is not nearly as substantial
a source of nitrogen as biological fixation, it is ubiquitous,
accounting for about 4% of the nitrogen available to the biosphere
(Bezdicek and Kennedy, 1998).
A more substantial mode of atmospheric nitrogen deposition
occurs due to combustion on land. Burning releases NOx
and other nitrogen-rich aerosols. Combustion of, primarily,
plants and plant detritus on land has occurred for hundreds
of millions of years. Little of the world's total vegetation
burned in a typical year, until a few thousand years ago.
The area burned every year, however, has substantially increased
in the past several thousand years due to altered land-use
practices by people. The area burned every year can even be
the majority of some countries' land area (e.g., Madagascar).
Combustion of vegetation accounts for approximately 8% of
the combined nitrogen in the biosphere, though only part of
this becomes available as aerosols in the atmosphere (Bezdicek
and Kennedy, 1998).
In addition, the combustion of fossil fuels, such as in car
engines, produces NOx. Catalytic converters substantially
reduce the amount of NOx released from cars by
catalyzing the conversion of NOx to N2
and O2 gases. Industrial applications can also
release large quantities of NOx, especially if
they have not installed or maintained pollution control devices
that limit the release of these substances. NOx
are, after all, major air pollutants causing illness and even
death for hundreds of thousands of people around the world
every year, especially when concentrated, as in metropolitan
or industrial areas. Atmospheric deposition of NOx
from industrial applications and motor vehicles accounts for,
on average, more than 20% of the total amount of fixed nitrogen
available to the biosphere (Bezdicek and Kennedy, 1998). This
deposition pattern is highly spatially heterogeneous, however.
That is, in areas far from anthropogenically produced sources
of NOx the contribution may be very small. For
example, a tiny island in the middle of the Pacific, outside
the areas of NOx fallout from industrialized countries,
might receive very little nitrogen from this source. On the
other hand, areas close to industry and population centers,
or "downwind" from them, so-to-speak, may receive
substantial nitrogen enrichment from this source. We might
therefore expect a coral reef off Sri Lanka to receive substantially
more nitrogen originating through atmospheric deposition than
Bikini atoll in the middle of the Pacific. At least a small
amount does reach every corner of the globe, though.
This large spatial heterogeneity is significant in determining
the impact of NOx on ecosystem function. If the
NOx were evenly spread over the entire globe, it
is unlikely it would cause major problems in many, if any,
of the world's ecosystems. However, because this is not the
case, the release of NOx has caused substantial
damage in some places due primarily to acid rain and secondarily
by the effects of dramatically higher nitrogen availability.
These problems have been most acute in forested ecosystems
with low soil alkalinity and freshwater ecosystems with low
alkalinity (soil and water alkalinity neutralizes acid rain
and ameliorates its effects).
It's Sprinkling…Phosphorus and Iron
As I mentioned in the first
article in this series, phosphorus lacks a significant
gaseous stage in the biosphere. This is in stark contrast
to the major pools of carbon and nitrogen in the atmosphere.
Iron, too, lacks a significant gaseous state in the atmosphere.
The air, however, does not stay still. Indeed, the sun's uneven
heating causes convection currents to travel through the atmosphere-otherwise
called wind. When blowing forcefully, wind has the power to
move even very large objects. However, even when weak, wind
has the power to pick up and carry dust and dirt, often over
substantial distances. What is in the dust and dirt that the
wind is constantly blowing around? You guessed it; among other
things, it contains phosphorus and iron. Just how much, and
how significant can this transport possibly be? Schlesinger
(1997) estimates the amount of phosphorus carried as dust
in the atmosphere to be on the order of 1.0 Tg P yr-1,
or about 1 billion metric tons of phosphorus per year. A billion
metric tons is a lot of anything. To be fair, not all of this
phosphorus will become biologically available and 1.0 Tg P,
while a lot, pales in comparison to the amount of phosphorus
actively cycled in ecosystems or available in most sediments.
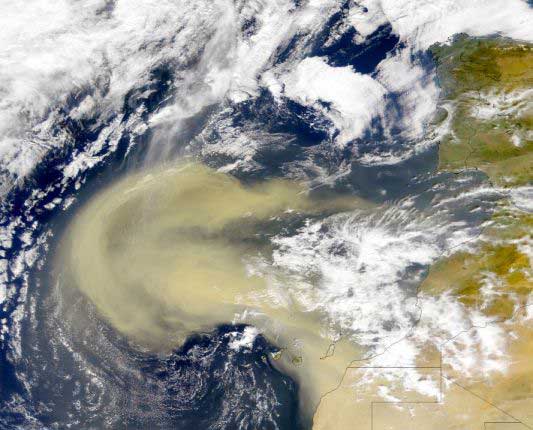 |
|
A satellite image captures a cloud of dust blowing off
Northern Africa.
Image courtesy NASA.
|
Just as with NOx deposition, the deposition of
P- and Fe-rich dust is quite heterogeneous over the globe,
with some places receiving substantially more dust than others.
The two regions that supply most of the dust in circulation
in the atmosphere are Northern Africa and the arid interior
parts of Asia, though atmospheric dust can originate from
any exposed land surface. This dust's deposition dramatically
affects the productivity of the ecosystems where it settles.
The subtropical and temperate Atlantic Ocean is relatively
productive compared to much of the ocean, thanks, in part,
to Saharan dust. As discussed in Part
II of this series, the Southern Ocean has very low rates
of productivity due to a lack of iron. This is due primarily
to prevailing wind patterns, which blow from the northwest.
The only land northwest of the Southern Pacific are tiny island
dots in an expanse of blue ocean. The Southern Ocean is Fe-limited
because it does not receive significant inputs of Fe-rich
dust, and it cannot receive these inputs because the wind
is simply blowing in the wrong direction-over thousands of
miles of ocean. The dust coming off Asia reaches some, but
certainly not all, of the islands in the northern and western
Pacific. This leads to substantially different natural soil
fertilities on different islands throughout the region. The
effect can be so dramatic that these differences may have
contributed to the perseverance or collapse of certain island
societies in the Pacific (Diamond, 2005).
Some Quintessence of Dust
The amount of atmospheric
dust in circulation has steadily increased since the last
Ice Age due to the increased area of arid land. At the height
of the last Ice Age much of the Sahara was covered in grassland
and scrub, similar to what much of sub-Saharan Africa looks
like today. As the climate warmed from ice age to pre-industrial
conditions, the arid central portion of Africa grew, replacing
grassland and scrub with desert. It is thought that land use
practices by people living in the region at the time may have
contributed to this transformation and caused the Sahara to
expand more widely than it otherwise would have, though it
is uncertain to what degree. Changes in land use by people
over the past century have dramatically increased the amount
of arid land area around the world (a process called desertification),
and these land use practices are projected to increase the
area of arid land even more substantially over this century.
As an example of how land use practices can affect the amount
of atmospheric dust in circulation, let us consider how the
major agricultural region of the United States became the
Dust Bowl during the 1930s. The Great Plains, like many grasslands,
are prone to occasional droughts. Starting in the 1920s the
Great Plains experienced what ended up as a decade-long drought.
The Southern Plains were much more affected than the Northern
Plains, but the drought affected the entire region. Normally
during years of drought, plants that can withstand the dry
weather survive, while plants that are less tolerant perish.
In general, however, the soil remains firmly locked in place
by the living plants' root systems, and wind erosion is minimal.
However, plowing this land leaves the soil completely exposed
to the wind. In years of average or above average rain in
the Great Plains crops such as wheat quickly rooted and grew
on the exposed surfaces, reducing topsoil's wind erosion to
a modest amount. Due to the drought the crops failed, and
they failed for about a decade. This left huge areas of topsoil
completely exposed, allowing extraordinarily high rates of
wind erosion and, hence, the Dust Bowl. Practices that lead
to similar erosion problems are still commonplace throughout
most of the world. This problem is particularly concerning
when the rate of erosion is factored against the rate of soil
formation. A few bad years may cause the loss of topsoil that
took literally thousands of years to form and that will take
thousands of years to replace. The increasing availability
of this dust may be good news for some marine phytoplankton
starved of phosphorus or iron (though the effect is not expected
to be that great), but it is pretty bad news for the six billion
people who rely on agriculture for food. With luck, more sustainable
land use practices that have already been devised will be
more widely utilized in the near future.
 |
 |
|
Parched earth led to massive wind erosion in the Dust
Bowl. Images courtesy of NOAA.
|
This dust can also have health consequences. Saharan dust
reaches all the way across the Atlantic. In fact, children
living in parts of Florida and the Caribbean have higher incidences
of asthma and other respiratory diseases than would be predicted
due to anthropogenic air pollution. Many people in these same
areas also have more problems with allergies than those living
in other regions. A significant contributing factor to these
problems, if not the major cause, is the high amount of Saharan
dust in the air. This dust may prove problematic for wild
organisms, too. Not only phosphorus and iron are deposited
with this dust, but anything else that was in the soil as
well. Aspergillosis, a disease affecting Caribbean sea fans
and gorgonians, is caused by a soil fungus of the genus Aspergillus.
One of the reservoirs for this fungus is Saharan dust. This
disease can be fatal, though recovery is also possible. The
incidence of this disease appears to have increased in recent
years throughout much of the Caribbean and tropical Atlantic
(Alker et al, 2001; Bruno et al, 2003; Geiser
et al, 1998; Hayes and Goreau 1998; Jolles et al,
2002; Kim and Harvell, 2002; Kim et al, 2000a; Kim
et al, 2000b; Nagelkerken et al, 1997a; Nagelkerken
et al, 1997b; Petes et al, 2003; Rosenberg and
Ben-Haim, 2002; Shinn et al, 2000; Smith et al,
1998; Smith et al, 1996).
In addition to the atmospheric deposition of terrestrial
dust, pollen is also a significant transporter of nutrients
through the air. A variety of plants (especially grasses and
temperate trees) rely on wind pollination for reproduction.
In order to be effective, however, these plants must produce
a significant excess of pollen beyond what they actually need
for reproduction. These "surplus" granules make
their way into worldwide circulation and can be a significant
source of phosphorus for certain oligotrophic ecosystems in
particular, though probably not most coral reefs. Crashing
ocean waves and a number of other processes can also create
aerosols of phosphoric and other salts, which are transported
by the atmosphere. These aerosols cause a loss of nutrients
such as phosphorus from the ocean, which can be deposited
either in a faraway part of the ocean or on land. Indeed,
the sky is made of much more than just air. Truly, it is a
thin soup consisting of gases, particles, aerosols and even
living organisms, and its circulation transports all of these
substances, sometimes over many thousands of miles.
Don't Forget Sulfur
A major connection between the ocean
(including coral reefs) and the atmosphere involves the element
sulfur. In order to form a droplet, water vapor must have
a nucleus on which to condense. In order to form a cloud,
there must be a lot of water droplets and, hence, a lot of
condensation nuclei. Over land, dust and aerosols from land
satisfy this need. Clouds form over the open ocean, too, and
this occurs far away from land where terriginous dust could
have a major influence. What is providing the nuclei for cloud
formation far from land? The surprising answer is phytoplankton.
Or, better said, the process of producing condensation nuclei
begins with phytoplankton.
A few types of aquatic plants and many types of algae, especially
coccolithophorids and dinoflagellates in the ocean, produce
a chemical called dimethylsulfoniopropionate (yes, there are
26 letters in that word and no, you don't need to memorize
it), or DMSP for short. This chemical has been implicated
in several metabolic processes. Upon death DMSP is quickly
converted to methanethiol and dimethyl sulfide (DMS) by bacteria.
Methanethiol tends to be used up by bacteria quickly to produce
sulfur-containing proteins (sulfur is present in the amino
acids cysteine and methionine and, hence, in proteins) while
DMS is used more slowly. Some DMS may be oxidized to dimethyl
sulfoxide (DMSO) while another portion escapes to the atmosphere
as an aerosol. This becomes oxidized to sulfate and provides
nuclei for cloud formation. During mass bleaching events,
when a large number of zooxanthellae (dinoflagellates of the
genus Symbiodinium) from corals and other symbiotic
organisms are dying, the substantial release of DMS may actually
induce cloud formation over the reef. Even during non-bleaching
events, however, there is turnover of zooxanthellae and other
algae on the reef, which releases DMS and has the potential
to affect local weather patterns (Hill et al, 2000;
Hill et al, 2004).
Sulfur aerosols (generally referred to as SOx-pronounced
"socks"…oddly enough) are also released by
industry and motor vehicles, and these sources account for
the major portion of SOx in the atmosphere at temperate,
northern latitudes. These SOx produce acid rain
just as NOx do. In the tropics, and especially
in the southern hemisphere, which has less land area and less
industry than the northern hemisphere, natural production
of SOx dominates. NOx and SOx
(not to mention volatile organic compounds, or VOx-no,
I'm not making these names up) originate from volcanic as
well as biological and industrial sources. The amount of these
substances in atmospheric circulation due to volcanism varies
from year-to-year, depending on volcanic activity. Human and
natural sources overshadow volcanic ones during most years,
though large volcanic eruptions such as of that of Mt. Pinatubo
in 1991, while infrequent, can be very important over the
short-term of several years.
Carbon Dioxide and Photosynthesis
A series of articles
could easily be written examining just the role of this gas
in influencing the ecology and biology of marine organisms.
Despite this I will try to curb my enthusiasm and report only
what I consider the essentials for appreciating carbon dioxide's
influence on reefs.
I hate to always refer to paleobiology in these discussions,
but I believe that it is instructive, so here we go. As the
first life arose on the planet the concentration of CO2
in the atmosphere was much higher than it is now. In fact,
at ca. 39.8 matm (milliatmospheres, 1/1000th of
an atmosphere) during the late Archaean and early Proterozoic
eons (2.75 - 2.2 billion years ago) it was about 100 times
greater than today's concentration (about 378 µatm)
(Rye et al, 1995). That is a lot of CO2!
Soon after this period photosynthetic life began to develop
and draw down this concentration. The earliest photosynthetic
organisms were cyanobacteria. They may be ugly as mats when
found growing in an aquarium or in nature, but they sure are
neat from an esoteric point of view.
The process of photosynthesis uses an enzyme to fix carbon
dioxide, along with water, into organic carbon compounds.
The enzyme is named ribulose-1,5-bisphosphate carboxylase/oxygenase
(another horrible name you needn't remember) which is usually
shortened to RuBisCO (rubisco) or even simply RuBP. The general
form of this reaction is:
CO2 + H2O + light à
CH2O + O2
This leaves the photosynthetic organism with organic carbon
that it can use for energy metabolism, for tissue growth and
repair, and for reproduction. However, as suggested by its
longer name, rubisco is not only a carboxylase but also an
oxygenase, meaning it can cause the reaction to proceed in
the reverse direction and burn up organic carbon. Ironically,
rubisco actually has a higher affinity for oxygen than for
carbon dioxide! This means that to work properly there must
be more CO2 than O2. For the first cyanobacteria
this wasn't a major problem because CO2 was abundant
and O2 was essentially absent from the atmosphere.
Today, however, oxygen constitutes 20.9% of the atmosphere
while carbon dioxide makes up just 0.0378%, 553 times less
than oxygen. The situation is a little bit better for aquatic
photosynthesizers. Oxygen is much less soluble than carbon
dioxide in water, and even less so in sea water, while carbon
dioxide is more soluble. In sea water at equilibrium with
the atmosphere at a salinity of 35 and a temperature of 25
C there will be about 206 µmol kg-1 O2
and about 10.78 µmol kg-1 CO2.
A factor of about 19 still separates the two, but this is
much less of a hurdle to overcome compared to the one that
land plants must negotiate. In order to allow the above reaction
to proceed correctly, modern CO2 users employ one
or several mechanisms to concentrate CO2 around
rubisco and, in many cases, mechanisms to quickly eliminate
oxygen. Many aquatic autotrophs also utilize bicarbonate as
a source of carbon dioxide. These organisms have the advantage
of bicarbonate being far more abundant than either oxygen
or dissolved carbon dioxide in sea water. At S = 35, t = 25
C and total alkalinity, TA = 2300 µmol kg-1
(considered average for the ocean), sea water contains about
1761 µmol kg-1 bicarbonate-about
8.5 times the amount of oxygen and about 163 times the amount
of carbon dioxide.
Some marine algae and seagrasses utilize dissolved CO2
primarily, though not necessarily exclusively. Many marine
algae use both dissolved CO2 directly and bicarbonate
indirectly as sources of CO2. For a more thorough
discussion of the use of carbon dioxide and bicarbonate by
marine autotrophs as a source of carbon I direct readers to
Randy Holmes-Farley's recent and excellent review of the subject
here.
Rising CO2 and Photosynthesis
As I discussed in the first part of
this series, the concentration of carbon dioxide in the atmosphere
rose over the last century and is expected to continue rising
at an accelerating rate over this century due primarily to
the burning of fossil fuels. Prior to the industrial revolution
the concentration of CO2 in the atmosphere was
280 µatm. Its concentration now is about 378 µatm,
an increase of more than 35%. The atmospheric concentration
is expected to reach 560 µatm, double the preindustrial
concentration, as early as 2065. Many CO2 users
such as land plants and seagrasses, because of the relatively
low abundance of CO2, are thought to be slightly
carbon-limited. Increasing the partial pressure of CO2
(pCO2) should therefore increase these plants'
rate of production. Indeed, increased rates of productivity
have been measured in a variety of Free-Air CO2
Enrichment (FACE) experiments, as well as in mesocosm and
laboratory studies. However, this increased productivity is
not necessarily manifested in a way that offers more food
to organisms that are higher up the food chain. For instance,
the increased rate of productivity in many land plants often
manifests as a disproportionate increase in belowground production.
In other words, the roots grow faster but the parts usually
eaten, and that provide most of the nutrients to the ecosystem,
may not grow much faster (Canadell et al, 1995). Increased
rates of productivity based on carbon may also prove not to
be useful or even to be detrimental due to the production
of lower quality food. Most plant foliage tends to be very
rich in C and poorer in N and P compared to the tissues of
autotrophs. Because autotrophs' tissue composition can vary
somewhat, depending on the availability of nutrients, increased
production based on carbon availability is likely to skew
the C:N:P ratio even higher in favor of carbon. Herbivorous
and omnivorous animals that already have a difficult time
extracting sufficient amounts of N, P and other nutrients
besides carbon from their diets may have a difficult time
coping with food of even lower quality. The nutrient stoichiometry
of food has already been shown to be a powerful structuring
agent in some communities (Jannicke Moe et al, 2005).
Because most reef algae, including zooxanthellae, can utilize
bicarbonate as a source of CO2, it is thought that
they are not generally carbon-limited. Thus, increased partial
pressure of CO2 (pCO2) should not stimulate
higher rates of primary production on coral reefs. Most studies
on corals (Burris et al, 1983; Goiran et al,
1996) and on reef assemblages (Leclercq et al, 2002;
Reynaud et al, 2003) have found that net production
does not increase, while Langdon and Atkinson (2005) found
a greater than 20% increase in net production of carbon in
a coral assemblage at pCO2 of about 790 µatm.
The previous studies used net oxygen production as a proxy
for net primary production, which is standard protocol, whereas
Langdon and Atkinson (2005) measured net production of carbon
directly. They found that net oxygen production did not change,
in agreement with the previous studies, though the net production
of carbon increased. This may be due to an increase in the
production of carbon-rich compounds by zooxanthellae, thereby
increasing the C:N and C:P ratio of photosynthate translocated.
As discussed with land plants above, if the net production
of carbon truly does increase in corals under increased pCO2
it may come at no nutritional benefit to the coral. This does
support the hypothesis that corals are generally carbon-limited
in natural sea water and may help explain a variety of observations
involving photosynthesis and calcification in corals.
Rising CO2 and Calcification
Elevated carbon
dioxide has been shown experimentally to reduce the rate of
calcification in coccolithophorids (a class of phytoplankton
important to oceanic productivity), foramaniferans, coralline
red algae, scleractinian corals and reef assemblages (reviewed
by Kleypas et al, 2006). Of particular interest to
reef aquarists, the calcification rate of reef communities
has been found to decrease by up to 65% (Langdon et al,
2000) from the rate at preindustrial pCO2 to the
projected pCO2 for 2100 of 700 µatm, though
the consensus estimate is near a 17-37% decrease (Gattuso
et al, 1999; Kleypas et al, 1999). This is a
level of carbon dioxide easily attained in reef aquaria at
night, even with significant aeration, or especially when
employing calcium reactors. The pH of the surface ocean (assuming
S = 35, t = 25 C, pCO2 = 378 µatm and TA
= 2300 µmol kg-1-a carbonate hardness of
about 6.6 dKH) is currently 8.20. If these parameters are
held constant but pCO2 is increased to 700 µatm,
the pH will fall to 7.98. Many aquariums dip below pH = 8.0
at night, and many, if not most, employing just a calcium
reactor to maintain calcium and carbonate experience pH =
8.0 or lower for significant periods of time. This may be
a very bad thing if our goal is to induce rapid calcification
in reef organisms, especially corals. I should mention that
aquarists (and most of the world) use a different pH scale
than oceanographers. Aquarists use what is called the NIST
scale, whereas oceanographers use either the total scale or
the seawater scale (these two are essentially the same). This
is significant because these scales arrive at different pH
values in sea water. The values I have reported are calculated
with the NIST scale used by aquarists, so no transformation
is needed to interpret these numbers. The total and seawater
scales tend to give values around 0.1-0.2 units lower than
the NIST scale (hence pH = 8.06 and 7.84 for current and future
pH, respectively, on the total scale).
 |
|
CO2 reduces calcification in corals like
this Porites porites.
Image courtesy NOAA.
|
The pH of sea water has been demonstrated to exert a major
influence on the calcification of many reef organisms, including
corals. In general, higher pH increases calcification while
lower pH decreases calcification, though a pH either too high
or too low will likely cause physiological stress to corals
and other reef organisms, not to mention making it very difficult
to stop spontaneous, abiotic precipitation of calcium carbonate
at high pH. For instance, it is doubtful that a coral or other
reef organisms would tolerate very high (say, above 11.0)
or very low (say, below 6.0) pH for any significant length
of time, even if such conditions could be provided. Therefore,
don't try replacing a few gallons of tank water with kalkwasser.
This probably will not lead to faster calcification rates
in your aquarium, but might be very effective at killing things.
Also, the pH on most coral reefs (especially in shallow areas
such as reef flats) is usually not constant over a
24-hour period. In fact, the pH on a reef flat may vary from
less than 8.0 to more than 8.6 within 24 hours with no significant
deleterious effects on the animals. Sometimes it is stressed
that all water parameters in a reef aquarium must be kept
very stable to have success. If this were true about pH (and
many other parameters), then nature would be failing miserably
at growing reef organisms. Reef animals can easily tolerate
a daily pH fluctuation. The reason for the fluctuation in
pH on reefs, just as in reef aquariums, is that during the
day the rapid consumption of CO2 by photosynthesis
reduces its concentration faster than it can diffuse into
the water from the atmosphere, while at night community respiration
with no consumption due to photosynthesis raises the amount
of CO2 in the water faster than it can diffuse
out to the atmosphere. Calcification also continues at night
in corals, though at about one-half to one-third its daytime
rate. This is significant because calcification produces about
0.8 mol CO2 for every mol CaCO3 deposited.
One difference between the chemistry of aquarium water and
sea water that must be considered is aquarium water's generally
higher total alkalinity (though recently there has been a
growing trend among some hobbyists to adopt a total alkalinity
nearer that of natural sea water (NSW) in the aquarium). Increasing
the alkalinity of sea water, for instance, through the addition
of sodium bicarbonate, has been shown to increase the rate
of calcification in corals (Marubini and Thake, 1999). Again,
this suggests that corals may be carbon-limited in NSW. An
alkalinity higher than that found in NSW has never been shown
experimentally to be deleterious. In addition, overwhelming
anecdotal evidence suggests that an alkalinity higher than
that of NSW is not necessarily deleterious to corals or other
reef organisms in the aquarium. Elevated ammonium and nitrate
have been implicated in reducing calcification in corals in
many studies. Marubini and Thake (1999) demonstrated that
the addition of bicarbonate allows corals to overcome the
deleterious consequences of elevated ammonium and nitrate
on calcification. Can maintaining a higher alkalinity in the
aquarium allow us to offset the effects of lowered pH? Depending
on CO2's mechanism in poisoning calcification,
the answer is maybe yes and maybe no.
The Saturation State Hypothesis
Currently, most
of the literature (reviewed by Kleypas et al, 2006)
suggests that the cause of reduced calcification in marine
organisms at elevated pCO2 is a reduction of calcium
carbonate's saturation state. Saturation state is described
by the equation:
W = [Ca2+][CO32-]/Ksp
where [Ca2+] is the concentration of calcium,
[CO32-] is the concentration of carbonate
and Ksp is the solubility product of aragonite
or calcite, depending on the form of calcium carbonate produced
by the calcifying organism (e.g., corals produce aragonite
so the Ksp of aragonite is used for them; coccolithophorids
produce calcite so the Ksp of calcite is used for
them). In sea water the concentration of calcium is highly
conserved, so it depends mostly on salinity. That is, if the
salinity is higher, the calcium concentration is higher because
the entire seawater solution becomes more concentrated, and
vice versa if the salinity is lower. The solubility product,
Ksp, varies depending on several factors (salinity,
pressure, temperature, etc.). Of these, temperature affects
Ksp the most (in a meaningful way) in the context
of a reef aquarium. Calcium carbonate, unlike most substances,
is actually less soluble at higher temperatures. Thus, as
temperature increases, Ksp decreases and W
increases. Changes in Ksp over a degree or two are relatively
minor, though. The carbonate concentration is determined largely
by alkalinity and pH. When pCO2 increases, it shifts
the equilibrium percentages of carbonate toward bicarbonate
and carbonic acid/CO2 as described by this equation:
CO2(aq) + H2O
↔
H2CO3 ↔
H+ + HCO3- ↔
2H+ + CO32-
Thus, increasing pCO2 decreases the concentration
of carbonate and the saturation state of calcium carbonate
if alkalinity is not adjusted. If pCO2 is increased
and alkalinity is also increased to the proper level, the
two offset each other in terms of their effects on the carbonate
concentration.
Current models for the calcification of calcareous algae
such as Halimeda suggest that the saturation state
hypothesis may adequately explain lower rates of calcification
at elevated pCO2. In these calcifiers photosynthesis
removes carbon dioxide, raising the pH and, thus, the carbonate
concentration and saturation state in a confined area, which
leads to the precipitation of calcium carbonate. The changes
in carbonate concentration have also been invoked to explain
changes in the calcification rate in corals and other marine
invertebrates as well. Physiological data suggest, however,
that corals take-up bicarbonate, not carbonate, from sea water.
Also, more than 70% of the carbon that corals use for calcification
may originate from metabolically produced CO2,
with the remainder being provided by seawater bicarbonate
(Furla et al, 2000). If the change in carbonate concentration
leads directly to reduced calcification in corals and other
marine invertebrates, then current models of calcification
must be dramatically modified and the mechanism of carbonate
transport must be identified. Paradoxically, Reynaud et
al (2003) found that at a temperature of about 28°
C (82.4° F) the rate of calcification in Stylophora
pistillata decreased by 50% at a pCO2 = 798
µatm (control at 470 µatm), while calcification
actually increased by 5% in corals maintained at about
25° C (77° F) under similar carbon dioxide concentrations.
This suggests to me a lot of regulation by the coral host,
or perhaps mechanisms at play that are not yet known.
On the other hand, many cellular processes are highly dependent
on pH, with many enzymes and processes being highly sensitive
to pH. It is very likely that corals use a Ca-ATPase enzyme
to pump calcium ions into the calcifying fluid in exchange
for protons (H+) (McConnaughey and Whelan, 1997).
If the external sea water's pH falls and the (H+)
concentration increases, does the calcium pump become less
efficient? Does some other rate-limiting step in calcification
(perhaps the production of the organic matrix) depend upon
a specific pH range? I wish I knew, but further investigation
is required to determine the mechanisms at work. Suffice it
to say that this is far from the final word on calcification
in marine organisms.
Conclusion
A constant and vital
exchange of materials occurs between the atmosphere and the
ocean. Nitrogen, phosphorus and iron that are transported
and deposited by the atmosphere into the water overlying coral
reefs is an important source of these nutrients to reefs and
helps them to sustain high rates of primary productivity and
thereby proper function. Carbon dioxide from the atmosphere
also dissolves into sea water
and provides carbon for photosynthesis. Many reef autotrophs
use not only dissolved CO2, but also derive CO2
from bicarbonate. While many reef algae may not be carbon
limited, corals which support both photosynthesis and calcification
(both processes require inorganic carbon) may be carbon-limited
at NSW levels. Corals calcify faster when the availability
of inorganic carbon in the external sea water increases due
an increase in total alkalinity. This is likely true for many
other marine calcifiers as well. When the amount of organic
carbon is increased due to increased pCO2 (and
the associated drop in pH), however, just the opposite is
observed. For marine calcifiers (considering only calcification
and not photosynthesis) it is neither the pCO2
nor the total alkalinity that is directly important to them.
Rather, it is the availability of the species of carbon they
use for calcification and the pH that actually affect their
calcification mechanisms. Raising the alkalinity in an aquarium
with depressed pH may be sufficient to offset a decrease in
calcification due to low pH in all marine calcifiers, or perhaps
only in some. In corals, the marine calcifiers of greatest
interest to most aquarists, it is not clear whether raising
alkalinity is sufficient to ameliorate the negative effects
of reduced pH. For that reason, it may be prudent to keep
the aquarium water's pH near or above that of natural sea
water. I would suggest that another take-home message should
be that we cannot separate the ocean or the aquarium water
from the air overlying it. Keep this in mind when stirring
up dust or introducing aerosols and noxious gases and fumes
in the vicinity of an aquarium, or when noxious substances
are used outside a home with open windows. Anything in the
air invariably ends up in the ocean or in the aquarium, for
better or worse.
|