|
Photosynthesis is
the process whereby organisms take in light energy and convert
it into useful chemical energy. It is a critically important
process in most reef aquaria, but one which most aquarists
pay little attention to, aside from the recognized importance
of having appropriate lighting. This article is the first
in a series that looks at photosynthesis in reef aquaria from
a chemical perspective. Such chemical issues, for example,
include how organisms get the raw materials for photosynthesis,
whether aquarists need to "supplement" those, how
organisms eliminate the "waste" products of photosynthesis,
what are the chemical implications of too much or too little
light, how calcification in corals and clams relates to photosynthetic
efficiency, what the biochemical machinery is for collecting
light and converting it into energy, and how organisms have
evolved these processes in relation to their natural habitats.
The answers to these questions can have
an important bearing on husbandry practices in ways that reef
aquarists might not have considered. In particular, topics
covered in this article include whether the pH or alkalinity
of an aquarium or a refugium might impact the rate of photosynthesis,
and whether aquarists should consider the availability of
carbon dioxide to photosynthesizing organisms.
The most simplified chemical equation describing
photosynthesis is:
carbon dioxide + water + light à
carbohydrate plus oxygen
or in a chemical formula:
CO2 + H2O à
CH2O + O2
This article deals primarily with the first
reactant in this equation, carbon dioxide. The processes leading
to the uptake of carbon dioxide by photosynthesizing marine
organisms are an active area of research, with most of the
relevant publications in this area being released only in
the past five years. It turns out that the symbiotic dinoflagellates
(zooxanthellae) inside corals and clams1
are a special case in terms of carbon dioxide acquisition
due to the surrounding host animal, as well as the significant
amount of calcification taking place in the same organism.
Because photosynthesis
and calcification may be chemically interrelated, the
special aspects of photosynthesis in symbiotic and calcifying
organisms will be detailed in a future article.
Freshwater aquarists caring for brightly-lit planted aquaria
have long known the
importance of CO2, and often add
carbon dioxide directly to the aquarium water in one way
or another to supply those tanks' substantial need for this
material. Reef aquarists, on the other hand, might have just
as much or more photosynthesis taking place, but rarely worry
about adding carbon dioxide. Why? That's one of the topics
to be detailed in subsequent sections of this article. The
answer is not that seawater contains more CO2 than
does freshwater, but rather that seawater contains other chemicals
that can, in some cases, be used to supply carbon dioxide.
The contents of this article are:
Introduction
Many organisms in a reef aquarium
rely on photosynthesis to survive. These include diatoms,
green hair algae, cyanobacteria, macroalgae, Tridacna
clams and most corals and anemones that aquarists maintain.
In the case of clams, corals and anemones, this photosynthesis
is actually carried out by symbiotic organisms (zooxanthallae)
that live within the tissue of the host animal. In every case,
however, the cells that photosynthesize need to incorporate
carbon dioxide somehow, and they excrete oxygen.
Sometimes obtaining adequate carbon dioxide is easy for photosynthesizing
organisms, and sometimes it is difficult, requiring them to
develop special mechanisms to obtain it rapidly enough. In
order to understand how this happens in a reef aquarium, it
is first necessary to understand what happens to carbon dioxide
when it dissolves into seawater.
Carbon Dioxide in Seawater
Carbon dioxide is an interesting molecule.
When it dissolves into water it can take a number of different
forms. Even the rate at which it can move between some of
these forms impacts how organisms must develop special mechanisms
to be able to take up enough during rapid photosynthesis.
Carbon dioxide is present at about 350 ppm in normal air.
It was lower in the past, and has been steadily rising for
the past 100 years or so, largely due to the burning of fossil
fuels. A liter of air weighs about 1.3 grams, so at 350 ppm
carbon dioxide, that liter of air contains about 0.00046 grams
(0.5 mg) of carbon dioxide. This very low amount, coupled
with the kinetic issues (i.e., the slowness) of carbon dioxide's
entry into seawater, explains why it is often difficult to
keep reef aquarium water aerated enough to keep the pH from
rising when processes such as photosynthesis or the addition
of limewater
consume carbon dioxide.
When a gas phase carbon dioxide molecule enters water, it
is initially hydrated to carbonic acid:
CO2 + H2O à
H2CO3
That hydration process is surprisingly slow because it's
an actual chemical reaction, as shown schematically below:
The time for half of the CO2 molecules added to
water to hydrate is on the order of 23 seconds. That rate
is slow enough that many organisms have developed enzymes
to speed it up. Carbonic anhydrase, for example, catalyzes
the hydration and the reverse reaction (dehydration) to allow
organisms to process carbon dioxide more rapidly. It is used
by a wide array of organisms, from algae to people. In people,
it is important in allowing carbon dioxide gas to be expelled
from the lungs. Without it, the carbonic acid in the lung
tissues would not convert rapidly enough to gaseous CO2
to permit it to be adequately expelled by breathing.
The carbonic acid that is formed when carbon dioxide hydrates
can then very quickly equilibrate into the water's carbonate
buffer system, converting into both bicarbonate and carbonate
by releasing protons (H+):
The conversions between carbonic acid, bicarbonate and carbonate
are much faster than the hydration of carbon dioxide and for
most purposes can be considered instantaneous. Consequently,
carbonic acid, bicarbonate and carbonate are in equilibrium
with each other at any given point in time. The primary factor
that determines the relative amount of each species at equilibrium
in seawater is the pH, with a small
temperature effect as well.
In order to assess whether an organism requiring CO2
could benefit from any of the forms besides CO2
itself, it is useful to understand how much of each is present
in seawater. Seawater contains about 670 times more unhydrated
carbon dioxide than the hydrated version (carbonic acid).
At most pH values attained in a reef aquarium, however, bicarbonate
is far more prevalent than carbon dioxide.
Using the known
pKa values for carbonic acid and bicarbonate in seawater,
we can proceed to determine exactly how much of each form
is present in seawater as a function of pH. The relevant chemical
equations and pKa values are:
CO2 + H2O ßà
HCO3- + H+ pKa
= 5.85
HCO3-ßà
CO3--+ H+ pKa
= 8.92
These pKa values imply that seawater at pH 5.85 contains
equal concentrations of carbon dioxide and bicarbonate, and
that seawater at pH 8.92 contains equal concentrations of
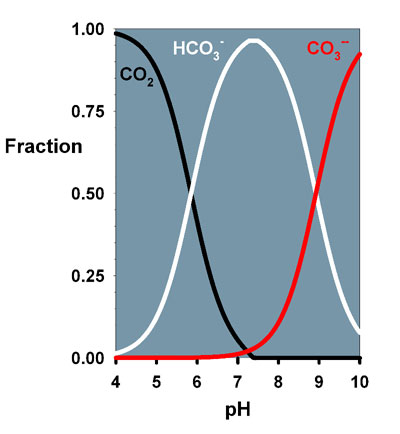
bicarbonate and carbonate. Figure 1 shows data calculated
for all three species as a function of pH in seawater. From
this graph, it is clear that if getting carbon dioxide itself
is limiting at pH 8.2, it might be more efficient to get it
from bicarbonate because so much more is present. In fact,
roughly 200 times more bicarbonate than carbon dioxide is
present in seawater at pH 8.2. In most reef aquaria the bicarbonate
is present at between 2 and 4 mM (millimolar = meq/L), or
about 122 to 244 mg/L bicarbonate. For comparison, carbon
dioxide is much lower, on the order of 0.01 mM (0.5) mg/L
at pH 8.2. Interestingly, that value of 0.5 mg/L for carbon
dioxide in seawater is almost exactly the same as the concentration
of carbon dioxide in air.
|
|
Figure 1. Relative fraction of carbon dioxide
and carbonic acid (black), bicarbonate (white) and carbonate
(red) in seawater as a function of pH.
|
Obtaining Carbon Dioxide as Carbon
Dioxide: Passive Uptake
Carbon dioxide is able to cross cell
membranes because it is a small uncharged molecule with reasonable
solubility in organic materials. Consequently, organisms that
take up carbon dioxide can do so passively (without spending
any energy) and with no special mechanisms (such as proteins
designed to speed up that process). Many marine algae and
other organisms take up some measurable portion of the carbon
dioxide that they incorporate during photosynthesis by this
process.
In most cases, however, this process can account for only
a portion of the demand for carbon dioxide. The rate at which
carbon dioxide is used by rapidly photosynthesizing organisms
is fast enough that organisms can deplete the carbon dioxide
in the surrounding seawater faster than it can be replaced
by diffusion and other transport mechanisms through the seawater.
The depletion is readily observed by the pH in the near surface
regions of these organisms, where the pH rises due to carbon
dioxide loss. For this reason many marine organisms have developed
other means of obtaining carbon dioxide, including processes
involving bicarbonate.2
Freshwater algae, on the other hand, can sometimes obtain
all of their required carbon dioxide by passive uptake.3
While a review of such literature is unnecessary in this article,
I'll give one example. The freshwater chrysophyte alga, Mallomonas
papillosa, has been shown to have none of the more sophisticated
mechanisms for carbon dioxide uptake that are described later
in this article, and it relies on simple passive uptake. For
this reason it has been shown to photosynthesize most effectively
where carbon dioxide concentrations are high, at pH 5-7.4
Obtaining Carbon Dioxide: Concentrating
Mechanisms
As mentioned above, few marine organisms
have been shown to rely solely on passive carbon dioxide uptake,
but the carbon dioxide concentrating mechanisms are often
unknown. As stated in a review article5
in 2005, marine diatoms fix more than 10 billion tons of carbon
by photosynthesis each year, but "there are still a number
of fundamental unresolved aspects of inorganic carbon assimilation
by marine diatoms. It is not clear how the carbon-concentrating
mechanism functions."
Obtaining Carbon Dioxide as Carbon
Dioxide: Active Transport
Carbon dioxide can be actively transported
across cell membranes by protein transporters. This process
does not solve the problem of low levels of available carbon
dioxide in the surrounding seawater, but it can ensure that
uptake itself is not a limiting factor, and may be especially
useful in environments where carbon dioxide is plentiful (implying
low pH environments in seawater).
The two marine dinoflagellates, Amphidinium carterae
Hulburt and Heterocapsa oceanica Stein, demonstrate
active uptake of carbon dioxide (or carbonic acid), but not
bicarbonate.6 Because this
mechanism is fundamentally limited in its effectiveness, it
has been speculated that these organisms may be CO2-limited
in their natural environment.7
Two marine haptophytes, Isochrysis galbana Parke and
Dicrateria inornata Parke, demonstrate active uptake
of both carbon dioxide (or carbonic acid) and bicarbonate
(described below).6,8
The marine diatom Skeletonema costatum9
has been shown to have little capability of using bicarbonate
to obtain carbon dioxide. It does, however, show active uptake
mechanisms for carbon dioxide, and this capability depends
on light levels. In higher light levels, the diatom shows
higher affinity for carbon dioxide. This capability can be
attained within two hours of exposure to high light, and slowly
fades over a period of about 10 hours when returned to low
light levels (where less carbon dioxide uptake is required).
Presumably, the organism is producing a carbon dioxide transport
protein when light levels are high and carbon dioxide is needed
in large amounts, and it halts that production (allowing the
transporters to slowly decline in population) when they are
not needed. High ambient levels of carbon dioxide also repress
the expression of its high affinity for carbon dioxide uptake.
Apparently, this diatom spends the energy to take up carbon
dioxide actively only when it is actually necessary to do
so, and relies on diffusion when it can.
Obtaining Carbon Dioxide from
Bicarbonate: Carbonic Anhydrase
If an organism is to obtain carbon
dioxide from bicarbonate, several potential processes are
available, and different organisms take different approaches.
In many cases, the exact mechanisms have not been established.
It is much easier to show that bicarbonate is a source of
carbon dioxide for marine organisms than to show exactly how
they take it up. A bicarbonate ion, being charged and insoluble
in organic phases, cannot readily diffuse across cell membranes,
so other mechanisms are needed.
Such uncertainty of mechanism is the case for Ulva lactuca,
for example. It has been shown to be able to photosynthesize
when out of the water (say, exposed at low tide), taking up
carbon dioxide directly, and also when in the water, taking
up bicarbonate.10 But the
exact mechanism of using bicarbonate to obtain carbon dioxide
isn't known in this species.
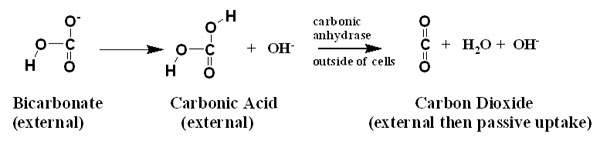
One common way to use bicarbonate is for the cells exposed
to the seawater to use extracellular carbonic anhydrase on
their surfaces. As mentioned above, the enzyme carbonic anhydrase
catalyzes the hydration and dehydration of carbon dioxide
and carbonic acid, respectively. These organisms present this
enzyme to the bicarbonate-rich seawater surrounding them.
Because the bicarbonate is naturally in rapid equilibrium
with carbonic acid, and the carbonic anhydrase keeps the carbonic
acid in rapid equilibrium with unhydrated carbon dioxide,
the bicarbonate is used as a ready pool to supply carbon dioxide
to passively cross cell membranes and be taken up (shown schematically
below).
 |
The agarophyte Gracilaria lemaneiformis11
has been shown to take up carbon in this fashion. It has carbonic
anhydrase both inside the organism and out. Inhibiting either
of these types of carbonic anhydrase greatly decreases photosynthesis,
but adding an anion transport inhibitor does not. Adding TRIS
buffer to the extracellular fluid (seawater) also has no effect
(the purpose of which is discussed in the following section
relating to proton pumping as a possible mechanism).
Photosynthesis in this organism is greatly reduced as the
pH is raised (73% reduction when going from pH 8.0 to 9.0),
presumably because the bicarbonate's propensity to form carbonic
acid is reduced at higher pH.
The brown alga, Hizikia fusiforme (Sargassaceae),12
from the South China Sea, has also been shown to exhibit carbonic
anhydrase activity, both inside and out, and has been shown
to be incapable of actively and directly transporting bicarbonate.
Consequently, its carbon dioxide concentration likely operates
by the mechanism shown above.
Two species of marine prymnesiophytes (Dicrateria inornata
and Ochrosphaera neapolitana)13
have been shown, through the use of various carbonic anhydrase
inhibitors, to use extracellular carbonic anhydrase to collect
carbon dioxide from ambient bicarbonate. They also employ
an energy dependent process for taking up carbon dioxide itself.
Growth in high carbon dioxide environments represses the expression
of carbonic anhydrase active in these species, but does not
reduce the active uptake of carbon dioxide.
Obtaining Carbon Dioxide from
Bicarbonate: Direct Uptake
An alternative way to obtain carbon
dioxide via seawater bicarbonate is to take up the bicarbonate
through protein transport mechanisms across the cell membranes,
and then once inside the cells where it is needed, carbonic
anhydrase converts it into carbon dioxide and hydroxide ion.
The hydroxide is then pumped out, or H+
is pumped in, to achieve pH balance.
Transporting ions across cell membranes using protein transporters
is a widespread mechanism whereby organisms can get needed
ions across a membrane through which they do not normally
diffuse. Some of these are active transporters, using chemical
energy to "pull" ions out of the extracellular fluid
(our push them out, as necessary), and other transporters
simply allow specific ions to pass though from high concentration
on one side to lower concentration on the other side.
The marine red alga Gracilaria conferta has been shown
to have an active bicarbonate uptake mechanism.14
Three marine bloom-forming (red tide) dinoflagellates, Prorocentrum
minimum, Heterocapsa triquetra and Ceratium
lineatum,15 have been
shown to take up bicarbonate directly. They show little carbonic
anhydrase activity, yet bicarbonate accounts for approximately
80% of the carbon dioxide they use in photosynthesis. It is
believed that these dinoflagellates are not carbon limited
in photosynthesis due to their efficient direct bicarbonate
uptake mechanisms.
The marine diatom Phaeodactylum tricornutum16
was found not only to have an active bicarbonate uptake mechanism,
but the researchers further identified at least two different
mechanisms. In particular, they showed that part of the uptake
depended on the presence of extracellular potassium, and this
part of the total carbon dioxide uptake was eliminated when
potassium was missing from the medium. A second direct bicarbonate
uptake mechanism was independent of potassium, indicating
the presence of at least two different pathways for transporting
bicarbonate into this organism.
Obtaining Carbon Dioxide from
Bicarbonate: Proton Pumping
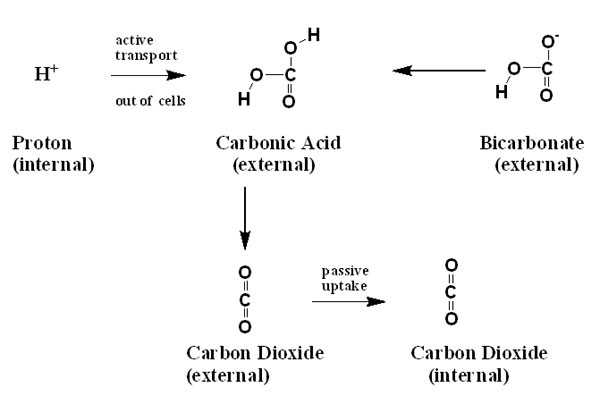
Another way
to obtain carbon dioxide via seawater bicarbonate is to pump
H+ out of the cells into
the extracellular fluid (seawater near the cells) or into
a special cavity where bicarbonate is present.17
This low pH causes the bicarbonate to become protonated to
become carbonic acid. The carbonic acid can then transform
into carbon dioxide, and pass across the cell membranes.
 |
The seagrass Zostera noltii Hornem18
has been shown, for example, to use proton pumping to gather
bicarbonate in the form of carbonic acid from the water. It
contains no extracellular carbonic anhydrase, but rather uses
ATP (adenosine triphosphate, the fundamental currency of chemical
energy in most organisms) to drive the export of H+.
Evidence for this mechanism is found by adding a buffer to
the seawater (TRIS) without changing the pH. This buffer keeps
the pH near the cell surface constant, counteracting the beneficial
effect of the proton pumping in lowering pH and converting
bicarbonate into carbonic acid. The simple presence of a non-absorbed
buffer in the water can decrease the rate of photosynthesis
in this organism by almost 80%.
Interestingly, those seagrass specimens acclimated to high
light (where high rates of photosynthesis and consequent uptake
of bicarbonate would be highest) showed the greatest ability
to actively take up bicarbonate. In high light experiments,
these previously high light-acclimated specimens were shown
to be only light limited, while the shade-acclimated organisms
were both light and carbon limited when put into high light.18
Other seagrass species (e.g., Z. mulleri and Z.
marina) have been shown to have external carbonic anhydrase,
and so may have different uptake mechanisms.18
Photosynthesis of Macroalgae
as a Function of pH
One of the side effects of the necessity
of taking up carbon dioxide to photosynthesize is that pH
may affect the rate of photosynthesis, because the amount
of carbon dioxide (as CO2 or H2CO3)
in the water varies with pH. Assuming constant carbonate alkalinity,
the effect is quite strong. A drop of 0.3 pH units implies
a doubling of the carbon dioxide concentration. A reef aquarium
at pH 8.5, for example, has one fourth the carbon dioxide
of a reef aquarium at pH 7.9, assuming the carbonate alkalinity
is the same.
Aquarists may rightly wonder whether organisms are able to
photosynthesize efficiently as the pH is raised. The answer
is mixed. Some can and some cannot. Those organisms that rely
solely on carbon dioxide may not. Those that rely on both
carbon dioxide and bicarbonate have a better chance of retaining
efficiency at higher pH because a much larger amount of bicarbonate
is present, and it does not change as rapidly with pH over
the range of interest to aquarists.
Table 1 shows the response of a variety of macroalgae in
terms of their ability to photosynthesize at pH 8.1 and 8.7.
In seawater with constant carbonate alkalinity, there is 20%
as much carbon dioxide at pH 8.7 as at pH 8.1, so an organism
relying on carbon dioxide alone might experience a large drop
in photosynthetic rate over this range. Clearly, the response
varies with species. Chaetomorpha aerea, in particular,
may be of substantial interest to aquarists. It is not necessarily
the exact species that many grow in refugia (which is unidentified
as far as I can tell), but this species of Chaetomorpha
shows a 25% drop in photosynthesis when exposed to the higher
pH. That drop is not as large as some other species, but may
still be important, and it is more than many other species
of macroalgae.
Of course, the photosynthesis rate does not necessarily translate
to growth rates. If other nutrients are limiting growth (nitrogen,
phosphorus, iron, etc.), then it may not matter if the rate
of photosynthesis is reduced at higher pH. But because these
nutrients are often present in surplus in reef aquaria, it
may well be that carbon uptake is limiting in some cases,
and in those cases aquarists might benefit from ensuring that
the pH is not too high.
| Species
of macroalgae: |
Relative
photosynthesis at pH 8.7 compared to pH 8.1 (as
a %):
|
| Chaetomorpha
aerea |
75
|
| Cladophora
rupestris |
100
|
| Enteromorpha
compressa |
67
|
| Ulva
rigida |
100
|
| Codium
fragile |
76
|
| Asparagopsis
armata |
45
|
| Gelidium
pusillum |
33
|
| Gelidium
sesquipedale |
18
|
| Gymnogongrus
sp.
|
39
|
| Osmunda
pinnatifida |
46
|
| Porphyra
leucosticta |
110
|
| Fucus
spiralis |
86
|
| Colpomenia
sinuosa |
100
|
| Dictyota
dichotoma |
53
|
| Cystoseira
tamariscifolia |
57
|
| Padina
pavonia |
53
|
|
|
Table 1. Relative rates of photosynthesis19
in seawater (measured by oxygen evolution) at pH 8.7
relative to pH 8.0. A value of 100 means that the rates
were the same, and values below 100 indicate less photosynthesis
at pH 8.7.
|
Photosynthesis of Algae Relative
to Their Natural Environment
Enough marine
algae have been studied with respect to carbon uptake to allow
certain comments about their capabilities to collect carbon
in relation to their natural habitat. In a study of 38 species
of red algae, researchers20
found that subtidal algae were more often restricted to using
carbon dioxide, while intertidal species could typically use
both carbon dioxide and bicarbonate. In fact, their ability
to use bicarbonate correlated strongly to their positioning
along a rocky coastline, with the efficiency increasing with
tidal height, except for those species at the very top of
the tideline, which showed a reversal of that trend. Similar
results have been found for other studies of macroalgae, including
green and brown algal species.21,22
Perhaps such relationships relate to the likelihood of spending
considerable time in small closed tidal pools, where carbon
dioxide would be more limited than in the open water, while
at the very top of the shore, where exposure to air is most
likely, the algae are again able to gain adequate carbon dioxide.
The actual mechanisms used by some species (the brown alga
Hizikia fusiforme,23
for example) that have multiple mechanisms actually change
when exposed to air (taking up CO2 directly) and
when immersed in seawater (using bicarbonate).
Photosynthesis of Algae in Continuous
Light vs. Light/Dark Cycles
Interestingly, three marine microalgae,
Skeletonema costatum, Phaeocystis globosa and
Emiliania huxleyi,24
were studied for their rates of photosynthesis and carbon
uptake mechanisms in continuous light vs. those same species
in light/dark cycles (12 h on/12 h off and 16 h on/8 h off).
The rates of photosynthesis were nearly twice as high with
light/dark cycles as with continuous lighting. In two of the
species (S. costatum and E. huxleyi), but not
the third, the contribution of bicarbonate to the total carbon
uptake increased dramatically in light/dark cycles compared
to continuous light.
How this result might relate to growth and nutrient uptake
in lit refugia where macroalgae are often grown to export
nutrients is not known. However, it is a sign that perhaps
continuous light is not optimal, in addition to being more
expensive.
Implications for Reef Aquarium
Husbandry
Aquarists can do several things to
ensure the ready availability of carbon dioxide to photosynthetic
marine organisms. While adequate studies have not been done
with the exact species and exact conditions present in a reef
aquarium to make definitive statements, it may be prudent
to follow the following principles in order to maximize photosynthesis:
1. Limit the maximum pH attained in reef aquaria. I'd suggest
limiting the pH to no more than 8.5, and lower is better from
a photosynthesis perspective (although pH
below 8.2 has its own disadvantages related to rates of
calcification by corals and coralline algae).
2. Put a lit refugium containing macroalgae on a reverse
light cycle to the main tank. Not only will this limit the
maximum pH attained in both the refugium and the main aquarium,
but it keeps the pH lower in the refugium precisely when the
organisms in it most need the pH to be lower (during the refugium
light cycle when CO2 is required).
3. Do not drip limewater (kalkwasser) into or upstream of
a lit macroalgae refugium, because it limits availability
of CO2.
4. Add the effluent of a CaCO3/CO2
reactor into or upstream of a lit macroalgae refugium, as
it increases the availability of CO2.
5. The data on continuous light vs. day/night cycling are
intriguing and suggest that a dark cycle may benefit lit macroalgae
refugia, especially when the cost of the electricity to drive
the lights is considered.
6. Keep the carbonate alkalinity up to at least 2.5 meq/l
(7 dKH; 125 ppm calcium carbonate equivalents) to provide
adequate bicarbonate for photosynthesis. Higher alkalinity
may even be better, especially if the pH is also high, limiting
carbon dioxide itself as a CO2 source for photosynthesizing
organisms. This suggestion is likely already followed by most
reef aquarists, but perhaps not by some with fish-only or
related types of aquaria that also rely on macroalgae for
nutrient export.
Summary
The availability of carbon dioxide
can be an important factor determining the rates of photosynthesis
in marine organisms. Even though bicarbonate is used by many
marine organisms, the ability of some species to photosynthesize
may be limited by the pH and the availability of carbon dioxide.
To be honest, before putting together this article, I had
not worried much about such issues. My system deviates from
several of the suggestions in the previous section (the pH
often hits 8.5 or a tad higher, the lighting is continuous
in three of my four refugia, etc.). I wonder what else I might
learn about how to care for my system as I explore additional
chemical aspects of photosynthesis in future articles?
Happy Reefing!
|