|
Magnesium is an
important ion in reef aquaria. Like calcium and alkalinity,
it can be depleted by various means if appropriate measures
are not taken to maintain it. In order to prevent its depletion,
or to correct a deficit when it occurs, magnesium supplements
are often used. These supplements can, however, be fairly
expensive, and this article describes how to make your own
magnesium supplements out of inexpensive materials available
to the public.
Magnesium's primary importance in reef
aquaria is its interaction with the calcium and alkalinity
balance. Seawater and reef aquarium water are always supersaturated
with calcium carbonate. That is, the solution's calcium and
carbonate levels exceed the amount that the water can hold
at equilibrium. How can that be? Magnesium is a big part of
the answer. Whenever calcium carbonate begins to precipitate,
magnesium binds to the calcium carbonate crystals' growing
surface. The magnesium effectively clogs the crystals' surface
so that they no longer look like calcium carbonate, making
them unable to attract more calcium and carbonate, so the
precipitation stops. Without the magnesium, the abiotic (i.e.
non-biological) precipitation of calcium carbonate would likely
increase enough to prohibit the maintenance of calcium and
alkalinity at natural levels.
For this reason, I suggest targeting the natural
seawater concentration of magnesium: ~1285 ppm. For practical
purposes, 1250-1350 ppm is fine, and levels slightly outside
that range (1200-1400 ppm) also are likely acceptable. However,
an aquarium's corals and coralline algae can deplete magnesium
by incorporating it into their growing calcium carbonate skeletons.
Many methods of supplementing calcium and alkalinity may not
deliver enough magnesium to maintain it at a normal level.
Settled
limewater (kalkwasser), in particular, is quite deficient
in magnesium, but so are many commercial materials sold for
use in calcium
carbonate/carbon dioxide (CaCO3/CO2)
reactors.
Consequently, magnesium should be measured occasionally,
particularly if the aquarium's calcium and alkalinity levels
seem difficult to maintain, or if there is excessive abiotic
precipitation of calcium carbonate on objects such as heaters
and pumps. If the magnesium level is found to be low, aquarists
can choose from a variety of commercial magnesium additives,
or they can use one of the DIY recipes described in this article.
The early sections of this article provide a background on
magnesium and explain why it becomes depleted. Later sections
describe the DIY recipes and show what impact they have on
the ionic balance in a reef aquarium over time.
The sections of this article are:
Magnesium in Seawater
Magnesium is the third most abundant
ion in seawater, behind sodium and chloride. It is also intimately
involved in a great many biological processes in every living
organism. Nevertheless, the situation that usually brings
it to most reef aquarists' attention is when it is suspected
of causing a problem with maintaining appropriate calcium
and alkalinity levels. In full strength seawater (salinity
= 35 PSU (= ppt)), magnesium is present at approximately 1285
ppm. Magnesium is about five times more abundant than calcium
on a per ion basis. Magnesium is significantly lighter than
calcium, so when compared on a weight basis, it is only about
three times as concentrated (1285 ppm vs. 420 ppm).
The magnesium content of seawater has not been constant since
the oceans formed. Specifically, the magnesium content has
often been lower than it is now, as in the late Cretaceous
period. As discussed below, the amount of magnesium getting
into calcium carbonate skeletons is a function of how much
magnesium is in the water. Consequently, the magnesium content
of ancient sediments can be significantly lower than more
modern ones from similar organisms. This fact may affect the
suitability of certain limestone deposits for maintaining
magnesium in aquaria, for example, when such limestone is
used in CaCO3/CO2 reactors.
Magnesium is present in seawater as the Mg++ ion,
meaning that it carries two positive charges, just as calcium
does. That, along with the fact that they have many other
chemical properties in common, is why calcium and magnesium
often displace each other in solid materials, such as coral
skeletons made of calcium carbonate with magnesium incorporated
into them in place of calcium.
Most of the magnesium in seawater is present as the free
ion, with only water molecules attached to it. It is estimated
that each magnesium ion has approximately eight water molecules
tightly bound to it. That is, water molecules that are so
tightly bound that they move with the magnesium ion as it
moves through the bulk of the water. For comparison, singly
charged ions such as sodium have only three or four tightly
bound water molecules. A small portion (about 10%) of the
magnesium is present as a soluble ion pair with sulfate (MgSO4),
and much smaller portions are paired with bicarbonate (MgHCO3+),
carbonate (MgCO3), fluoride (MgF+),
borate (MgB(OH)4+) and hydroxide (MgOH+).
While these ion pairs comprise only a small portion of the
total magnesium concentration, they can dominate the chemistry
of these other ions. An extended discussion of these facts
is beyond the scope of this article, but it should be noted
that these ion pairs can have huge impacts on seawater's chemistry.
In the case of carbonate, for example, the ion pairing to
magnesium so stabilizes the carbonate that it is present in
far higher concentrations than it would be in the absence
of magnesium. This effect, in turn, makes seawater a much
better buffer in the pH range of 8.0-8.5 than it otherwise
would be. Without this ion pairing, seawater's pH might be
significantly higher and more susceptible to diurnal (daily)
swings.
The average residence time for a magnesium ion in seawater
is on the order of tens of millions of years. That time is
substantially longer than that of calcium (a few million years)
and aluminum (100 years), but less than sodium (about 250
million years). In a certain sense, this is an indication
of how reactive magnesium is: it stays in seawater a long
time because it's fairly unreactive, but it does get taken
out of solution through various biological and chemical processes
more readily than does sodium.
Another interesting characteristic of ions is whether they
are excluded from organisms, actively taken up or just "allowed"
to be present. Like two other common ions, sodium and sulfate,
the magnesium concentration in organisms is approximately
the same as in seawater (not counting magnesium in skeletons).
This probably results from the facts that plenty of magnesium
is present in seawater, and that it is used by organisms for
many purposes. Chloride, another very common ion, is actively
rejected by organisms, and most other ions are substantially
concentrated.
The Effect of Magnesium on the
Calcium/Alkalinity Balance in Aquaria
How does magnesium impact the balance
of calcium and alkalinity in reef aquaria? Answering this
question requires a basic understanding of the calcium and
carbonate systems in seawater. I have detailed these in both
complex
mathematical ways, and in intuitive,
simplified ways. In short, calcium carbonate (CaCO3)
is supersaturated
in seawater, meaning that given enough time calcium ions
will interact with carbonate ions and precipitate as calcium
carbonate. If the concentration of either ion is pushed too
high, CaCO3 will start to precipitate. Magnesium
interferes with this process, permitting both calcium and
carbonate to be elevated above their level in the absence
of magnesium.
How does magnesium interfere with the precipitation of CaCO3?
The primary way involves magnesium "poisoning" the
growing CaCO3 crystals' surface, thereby slowing
the precipitation. It can, in fact, be slowed to the point
where it simply does not happen at rates problematic to an
aquarist.
In short, while magnesium carbonate is not supersaturated
in seawater (or in typical reef aquaria), and will not precipitate
on its own, magnesium is attracted to calcium carbonate surfaces
where the carbonate ions are already held in place by the
calcium ions. With the carbonate ions held in place, magnesium
finds it an attractive place to bind.
After a short time in seawater, a virgin calcium carbonate
surface quickly obtains a thin coating of Mg/CaCO3
(magnesian calcite) as magnesium pushes its way into, and
onto, the crystal's surface. Eventually, the surface contains
a substantial amount of magnesium. The extent to which this
occurs depends on the underlying mineral and is apparently
much more extensive on calcite than aragonite. It also depends
upon the water's relative amounts of calcium and magnesium.
Regardless, a new type of material is formed that contains
both calcium and magnesium.
This new mineral surface containing both calcium and magnesium
is not a good nucleating site for the precipitation of additional
calcium carbonate (as either aragonite or calcite), so the
precipitation of additional CaCO3 slows down substantially.
The importance for aquarists is that if the magnesium
concentration is too low, it may not adequately play this
role. In that situation, pumps and heaters (and any warm object)
may get coated with precipitating calcium carbonate. Further,
that precipitation removes calcium and alkalinity from the
aquarium, making it harder to maintain adequately high levels
of these two parameters.
Organisms that Use Magnesium
In terms of the amount of magnesium
consumed, the primary sink for magnesium in reef aquaria is
calcification. When calcium carbonate skeletons are deposited,
magnesium often gets into them in place of calcium. It is
not entirely clear whether this is something that organisms
"try" to control or not. Nevertheless, the amount
of magnesium entering the skeletons of different organisms
varies greatly.
How Much Magnesium Do Corals Consume?
The amount of magnesium incorporated
into the skeletons of various calcifying organisms varies
considerably. In a previous
article I showed that corals in the ocean can incorporate
between about 0.1% and 3.5% magnesium by weight in their skeletons
(Table 1). Coralline algae also incorporates a considerable
amount, typically more than 1%, and as high as 4.4%, by weight.
Few data are available on coral skeletons in aquaria, but
their magnesium content is not expected to differ significantly
from the same organisms living in the ocean.
Interestingly, coralline algae that normally pack a large
amount of magnesium into their calcium carbonate deposits
(>1% magnesium by weight) have been shown to incorporate
less magnesium when the water's magnesium content is reduced.
The amount incorporated is directly proportional to the magnesium
concentration. Consequently, the amount of magnesium that
they consume in aquaria depends on the water's magnesium content.
This effect is also likely to extend to other calcifying organisms
as well.1
In addition to the magnesium used in the process of calcification,
many (if not all) organisms take it up directly from seawater.
Check out Countdown Mailer for the specials of the week.
Organisms ranging from bacteria8-10 to fish11
take up magnesium, but the amount is generally much smaller
than that used during calcification. In many cases, there
is so much magnesium in seawater that the organisms expend
more effort pumping excess magnesium back out than they do
trying to take it up.
|
Table
1. Magnesium in Calcium Carbonate Skeletons |
| Organisms
|
Magnesium
content of skeleton (weight %) |
Reference |
| Corals: |
|
|
| Suborder
Asterocoeniina and Faviina |
0.07
- 0.36% |
2 |
| Suborder
Fungina |
0.095
- 1.22% |
2 |
|
Fungia actiniformis
var. palawensis |
0.091% |
6 |
| Suborder
Caryophylliina |
0.18
- 0.21% |
2 |
| Suborder
Milleporina |
0.12
- 0.53% |
2 |
|
Millepora sp. |
0.12
- 0.53% |
2 |
| Suborder
Stolonifera |
2.98
- 3.52% |
2 |
|
Family Tubiporidae |
2.98
- 3.52% |
2 |
|
Tubipora rubrum |
2.98
- 3.52% |
2 |
|
Family Dendrophylliidae |
0.05% |
2 |
|
Family Porites |
0.095
- 1.22% |
2 |
|
Porites lobata |
0.40
- 1.22% |
2 |
|
Family Pocillopora |
0.34% |
2 |
|
Family Dendrophyllia |
0.05% |
2 |
| |
|
|
| Gorgonia:
|
|
|
| Eunicella
papillosa, E. alba, E. tricoronata,
and Lophogorgia flamea |
2.2
- 2.7% |
5 |
| |
|
|
| Other
Organisms: |
|
|
| Coralline
algae in general |
>1% |
1 |
| Coralline
algae: Lithophyllum and Lithotamnium |
2.0
- 2.8% |
7 |
| Calcareous
alga: Corallina
pilulifera |
4.4% |
4 |
| Benthic
marine Ostracoda (crustaceans) |
0.5
- 1.3% |
3 |
|
Magnesium Consumption Relative
to Calcium
Calcium is present in the coral skeletons
described in Table 1 at about 35 - 38% by weight, because
they are largely calcium carbonate. Consequently, the Mg/Ca
ratio ranges from about 0.0025 to 0.12 by weight in corals.
Consequently, for a calcium supplement to be the sole source
of magnesium in an aquarium, it would have to include approximately
this same Mg/Ca ratio (0.0025 to 0.12) to preclude magnesium's
buildup or depletion over time. Obviously, with such a wide
range, the exact balance in any given aquarium will be determined
in part by the mix of corals and coralline algae being maintained.
Fortunately, there is such a large reservoir of magnesium
in seawater that it takes large differences between import
and export to cause important changes in magnesium levels.
Models of Magnesium Depletion
In order to understand what happens
over time to magnesium levels in an aquarium using an additive
that adds little or no magnesium, such as limewater, or a
DIY two-part system without the magnesium portion, I have
developed some simple models. This model assumes that no magnesium
is lost due to any process except calcification, and that
no magnesium is added. This model also assumes that magnesium
is removed from the aquarium as a co-precipitate with calcium
carbonate at an average level of 1% magnesium by weight (a
Mg/Ca ratio of about 0.025). An aquarium with a heavy load
of organisms that use more magnesium, like coralline algae,
may show a larger depletion of magnesium.
Table 2 shows what happens to magnesium over time when the
aquarium is supplemented daily with 4, 8 and 16 ppm calcium
and 0.2, 0.4 or 0.8 meq/L (0.6, 1.1, or 2.2 dKH) alkalinity.
For comparison, this dosage is equivalent to 0.5, 1 and 2%
of the tank's volume daily in saturated limewater.
|
Table
2. Magnesium Depletion Over the Course of a Year
with Dosing of Calcium and Alkalinity Only |
|
Daily
Alkalinity (meq/L) |
Daily
Calcium (ppm) |
Starting
Magnesium (ppm) |
Magnesium
Removed (ppm) |
Final
Magnesium (ppm) |
|
0.2 |
4 |
1280 |
37 |
1243 |
|
0.4 |
8 |
1280 |
74 |
1206 |
|
0.8 |
16 |
1280 |
149 |
1131 |
|
Models of Magnesium Depletion:
CaCO3/CO2 Reactors
In previous articles I have used published
data to show how magnesium
and strontium
might become depleted
over time in aquaria using calcium carbonate/carbon dioxide
reactors. Table 3 shows the relative concentrations of calcium
and magnesium in these different systems. The various reactor
media vary somewhat in their ability to maintain magnesium
if it is depleted at a Mg/Ca ratio of about 0.0025 to 0.12.
Because these media are somewhat deficient in magnesium, it's
anticipated that they will result in a long-term depletion
of magnesium, although not as rapidly as when no magnesium
is supplied (Table 2).
|
Table
3. Relative Concentrations of Calcium and Magnesium
in Different Reactor Media |
| Supplement: |
|
| Koralith
CaCO3 |
0.0024 |
| Super
Calc Gold CaCO3 |
0.0070 |
| Quarried
Limestone |
0.010 |
| Nature’s
Ocean crushed coral |
0.0065 |
| |
|
| A
Typical Coral |
0.025 |
|
It has been suggested that adding dolomite to CaCO3/CO2
reactors can help with magnesium problems. Dolomite is a material
that contains both magnesium and calcium carbonate. If dolomite
is added to the reactor to maintain existing appropriate magnesium
levels against their continual depletion via calcification
(for example, if the calcium carbonate used is too low in
magnesium to maintain adequate magnesium), then this is a
fine approach. Aquarists might typically use on the order
of 10% of the media as dolomite (and 90% as calcium carbonate).
However, this method is unsuitable if the goal is to
raise magnesium levels. The problem is that for every
magnesium ion released from the dolomite, two units of alkalinity
are also released:
MgCO3 à
Mg++ + CO3--
Consequently, raising magnesium in this way by 100 ppm will
necessarily raise the alkalinity by 8.2 meq/L (23 dKH). The
only way around this problem is to add a mineral acid (not
vinegar) to the aquarium to reduce the alkalinity, and that
may be more problematic than just adding a magnesium supplement
in the first place.
Toxicity of Elevated or Depleted
Magnesium
Very few studies have examined the
toxicity of elevated or depleted magnesium to most marine
organisms. In large part this circumstance likely stems from
the fact that magnesium in the ocean would almost never be
greatly increased or decreased in concentration, aside from
effects stemming only from salinity changes. I have summarized
what little seems to be known in a previous
article.
Sources of Magnesium in Marine
Aquaria Other than Supplements
The obvious primary source of magnesium
in marine aquaria is the artificial or natural seawater used
to set up the aquarium, and with which any water changes are
performed. Some artificial salt mixes have been reported to
be deficient
in magnesium, while others have been reported to have
a substantial
excess. Dissolution of calcium carbonate substrates containing
magnesium may provide a small amount of magnesium to a typical
reef aquarium, but not enough to maintain adequate levels
since such dissolution is fairly slow (normally not enough
to maintain adequate calcium or alkalinity levels either).
Another potential source of magnesium is fish food. Magnesium
is present in many foods at fairly high concentrations, but
not enough to significantly impact typical magnesium levels
(~1285 ppm). The effect of foods over the course of a year
has been estimated to be a boost of about 1-14
ppm magnesium.
Sinks for Magnesium Other than
Calcification
The primary sink for magnesium in
reef aquaria is co-precipitation with calcium carbonate (i.e.,
calcification). This occurs in organisms, as shown in Table
1, and also during the abiotic (i.e., non-biologically driven)
precipitation of calcium carbonate (such as on heaters and
pumps).
A potential sink that has been described by some hobbyists
is the precipitation of magnesium by limewater (kalkwasser).
Both magnesium hydroxide and magnesium carbonate have been
suggested as sinks for magnesium. I do not believe that either
is an important process in most aquaria. Adding any high pH
additive, including limewater, results in the transient
formation of magnesium hydroxide. This material quickly
redissolves upon mixing such that the local pH drops below
about 8.6.-9.0. Magnesium carbonate is a more complicated
issue, as it is near its solubility limit in seawater and
may quickly get coated with less soluble magnesium calcite.
These issues have been dealt with in a previous
article of mine as well as one by Bingman,
and the conclusion in both cases is that neither of these
precipitates likely is a long-term sink for magnesium in most
reef aquaria.
Supplements for Magnesium in
Marine Aquaria
A variety of commercial magnesium
supplements are available. Those supplements made by ESV and
Kent are quite popular, although I've seen no detailed analyses
of them. Assuming they are what they claim to be, they are
fine products to use, even for obtaining large increases in
magnesium levels in reef aquaria. I've used the ESV supplement,
along with ones that I've made myself. I would not suggest
raising magnesium by more than 100 ppm per day, in case the
magnesium supplement contains impurities. If the magnesium
level needs to be raised by several hundred ppm, spreading
the addition over several days will allow you to more accurately
reach the target concentration, and might allow the aquarium
to handle any impurities that the supplement contains.
One thing to keep in mind about magnesium supplements, including
the DIY recipe given below, is that they are all necessarily
quite "dilute" even when presented as dry solids.
The reason for this is that magnesium is a doubly-charged
and very light ion. So in salt form, or when dissolved in
a liquid, it is necessarily attended by a large number of
quite heavy counterions (chloride and sulfate, usually). Commercial
dry supplements may be only 8% magnesium by weight, for example.
There is just no way around that.
Compounding this issue is the simple fact that there is so
much magnesium in an aquarium that significant supplementation
requires a great deal of material. A 100-gallon aquarium contains
about a pound of magnesium! Raising that same aquarium's magnesium
level by 200 ppm would require the addition of on the order
of two pounds of dry magnesium salts!
DIY Recipes
The goal of any do-it-yourself (DIY)
recipe should be to allow safe and inexpensive supplementation
without negatively impacting the seawater's chemistry. Aquarists
can select either of two main materials to make such supplements,
and three recipes are appropriate to make from these materials.
1. Epsom salts (USP grade magnesium
sulfate heptahydrate) is readily available in drug stores
and is fairly inexpensive. The problem is that if this were
used to raise magnesium by a large amount (or a small amount
several times) the aquarium water would become enriched in
sulfate. This enrichment may not be a problem for some aquaria,
especially those using salt
mixes already deficient in sulfate, or those that employ
frequent water changes. Nevertheless, Epsom salts alone is
not an ideal magnesium supplement.
Table 4 shows the effect on an aquarium's sulfate level over
time of using only Epsom salts to supplement magnesium, and
when no water changes are employed. Clearly, the sulfate rises
quite substantially over time. Figures 1-6 show the effect
graphically with different water change amounts including
0%, 10%, 20% and 30% monthly, and 1% daily. In these cases,
sulfate rises, but water changes can help mitigate the rise.
If at least 30% is changed monthly, or 1% daily, using Epsom
salts alone as a magnesium supplement may be acceptable; otherwise
the sulfate buildup is likely too large to be optimal.
|
Table
4. Sulfate Rise Over the Course of a Year When
Using Magnesium Sulfate to Supplement All Magnesium.
(Results are
corrected for salinity changes.) |
|
Daily
Alkalinity (meq/L) |
Daily
Calcium (ppm) |
Starting
Sulfate (ppm) |
Final
Sulfate (ppm) |
Sulfate
Rise (%) |
|
0.5 |
10 |
2710 |
3034 |
12% |
|
1 |
20 |
2710 |
3353 |
24% |
|
1.5 |
30 |
2710 |
3668 |
35% |
|
[Note that these sulfate
rise values are lower, for a given calcium and alkalinity
dosage rate, in this model than I have shown previously for
my DIY
two-part additive recipe. The reason for this difference
is that in that case, additional magnesium sulfate is required
to offset the rise in sodium and chloride that comes along
with the calcium chloride and baking soda. Such differences
are unimportant for typical aquarists to understand, but this
explanation is provided for those who noted and were confused
by the difference.]
|
Figure 1. The rise in sulfate over time in an
aquarium with a daily dosage rate of 10 ppm calcium
and 0.5 meq/L (1.4 dKH) alkalinity. This dosage rate
is typical of an average mixed reef aquarium. Magnesium
is assumed to be supplied by using Epsom salts alone.
The various curves reflect different water changes scenarios,
including none (black), 5% monthly (green), 10% monthly
(yellow), 20% monthly (blue), 30% monthly (pink) and
1% daily (red). |
|
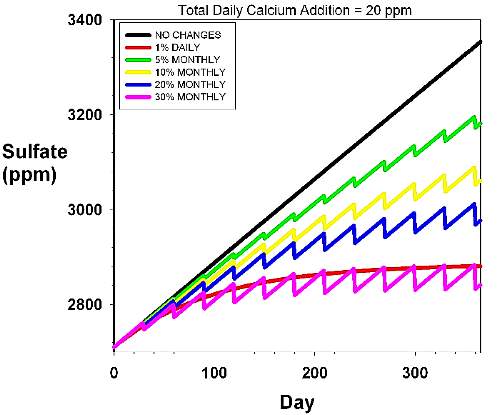
Figure 2. An expanded version of Figure 1, shown
to provide a more accurate assessment of the relative
rise of sulfate over the background concentration (2,710
ppm). The rise in sulfate over time in an aquarium with
a daily dosage rate of 10 ppm calcium and 0.5 meq/L
(1.4 dKH) alkalinity. Magnesium is assumed to be supplied
by using Epsom salts alone. The various curves reflect
different water changes scenarios, including none (black),
5% monthly (green), 10% monthly (yellow), 20% monthly
(blue), 30% monthly (pink) and 1% daily (red). |
 |
|
Figure 3. The rise in sulfate over time in an
aquarium with a daily dosage rate of 20 ppm calcium
and 1.0 meq/L (2.8 dKH) alkalinity. This dosage rate
is typical of a reef aquarium with a medium to high
calcification rate. Magnesium is assumed to be supplied
by using Epsom salts alone. The various curves reflect
different water changes scenarios, including none (black),
5% monthly (green), 10% monthly (yellow), 20% monthly
(blue), 30% monthly (pink) and 1% daily (red). |
 |
|
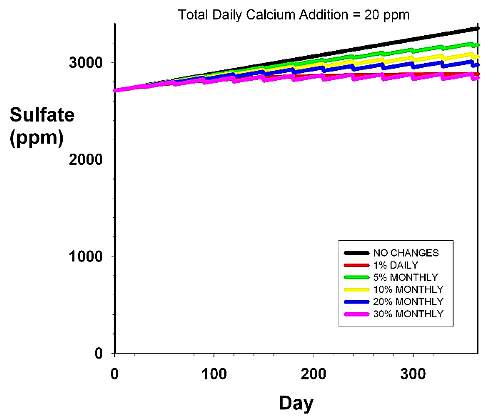
Figure 4. An expanded version of Figure 1, shown
to provide a more accurate assessment of the relative
rise of sulfate over the background concentration (2,710
ppm). The rise in sulfate over time in an aquarium with
a daily dosage rate of 20 ppm calcium and 1.0 meq/L
(2.8 dKH) alkalinity. Magnesium is assumed to be supplied
by using Epsom salts alone. The various curves reflect
different water changes scenarios, including none (black),
5% monthly (green), 10% monthly (yellow), 20% monthly
(blue), 30% monthly (pink) and 1% daily (red). |
 |
|
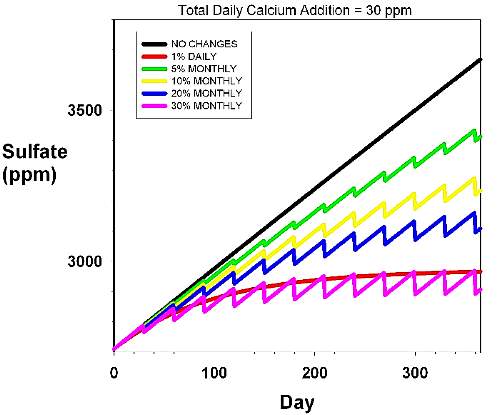
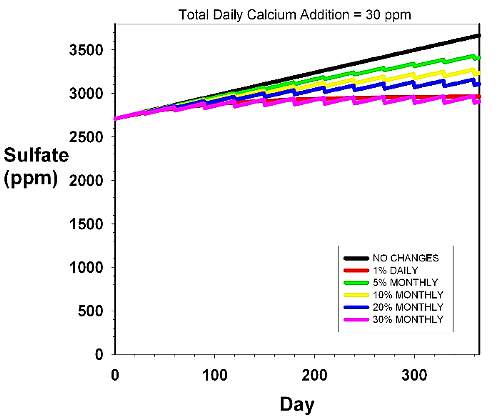
Figure 5. The rise in sulfate over time in an
aquarium with a daily dosage rate of 30 ppm calcium
and 1.5 meq/L (4.2 dKH) alkalinity. This dosage rate
is typical of an small polyped stony coral-dominated
aquarium with a high calcification rate. Magnesium is
assumed to be supplied by using Epsom salts alone. The
various curves reflect different water changes scenarios,
including none (black), 5% monthly (green), 10% monthly
(yellow), 20% monthly (blue), 30% monthly (pink) and
1% daily (red). |
 |
|
Figure 6. An expanded version of Figure 1, shown
to provide a more accurate assessment of the relative
rise of sulfate over the background concentration (2,710
ppm). The rise in sulfate over time in an aquarium with
a daily dosage rate of 30 ppm calcium and 1.5 meq/L
(4.2 dKH) alkalinity. Magnesium is assumed to be supplied
by using Epsom salts alone. The various curves reflect
different water changes scenarios, including none (black),
5% monthly (green), 10% monthly (yellow), 20% monthly
(blue), 30% monthly (pink) and 1% daily (red). |
It's also possible to determine the effect on sulfate from
one-time boosts to magnesium using Epsom salts. Epsom salts
actually contain about four times as much sulfate as magnesium,
so boosting magnesium gives an even larger boost to sulfate.
Table 5 shows the effect on sulfate of several different increases
in the magnesium level. From that table, it is clear that
boosting magnesium by 100 ppm or more using Epsom salts has
a substantial effect on the sulfate level. I therefore do
not recommend using this method to boost magnesium by more
than about 50-100 ppm. Every subsequent addition will compound
the effect, although water changes will serve to reduce it
over time.
|
Table
5. Sulfate Increase from a Single Magnesium Boost
Using Magnesium Sulfate.
(Results are
not corrected for salinity changes.) |
|
Magnesium
Boost (ppm) |
Starting
Sulfate (ppm) |
Final
Sulfate (ppm) |
Sulfate
Rise (%) |
|
50 |
2710 |
2908 |
7% |
|
100 |
2710 |
3105 |
15% |
|
200 |
2710 |
3500 |
29% |
|
300 |
2710 |
3895 |
44% |
|
2. A second DIY material is magnesium
chloride. Some grades of magnesium chloride traditionally
have been contaminated in ways that would preclude their use
in aquarium applications. They sometimes contain ammonia,
for example. So any random magnesium chloride brand that's
selected may not be acceptable. However, magnesium chloride
hexahydrate from the Dead Sea Works seems to be adequately
pure for this purpose. It is sold as a deicer (MAG flake
from hardware stores, for example) or as a dust control agent
for equestrian arenas.
Editors note (3/10/07): Note, the manufacturer of MAG flake has alerted us that they very strongly recommend against using this product in reef aquaria. While many reef aquarists have successfully used the product, the manufacturer does not claim to be able to provide this product at suitable quality in the future. |
As an alternative source of magnesium chloride, some aquarists
have begun to use Nigari,
a Japanese product derived from seawater that is used to manufacture
tofu. It appears to be mostly magnesium salts of chloride
and sulfate, but how much sulfate and how much chloride, as
well as what other metals it contains, remains to be demonstrated.
The recipes here will focus on MAG flake, but if you want
to experiment, substituting Nigari for MAG flake may be acceptable.
When used as a supplement to boost magnesium, magnesium
chloride will slowly boost the chloride level, but because
chloride is present in seawater at more than 19,000 ppm naturally,
a small boost is seldom important. Table 6 shows the rise
in chloride concentration over time using this supplement.
The chloride rises a bit over a year, but not enough to become
a concern. This rise is much smaller than the reported typical
variations between salt mixes (more than 1000 ppm chloride).
Any water changes will further mitigate this increase. I use
this material to boost the new artificial seawater that I
use (Instant Ocean) by 150 ppm magnesium.
|
Table
6. Chloride Rise Over the Course of a Year When
Using Magnesium Chloride to Supplement All Magnesium.
(Results are corrected for salinity changes.) |
|
Daily
Alkalinity (meq/L) |
Daily
Calcium (ppm) |
Starting
Chloride (ppm) |
Final
Chloride (ppm) |
Chloride
Rise (%) |
|
0.5 |
10 |
19,350 |
19,418 |
0.4
% |
|
1 |
20 |
19,350 |
19,484 |
0.7
% |
|
1.5 |
30 |
19,350 |
19,550 |
1
% |
|
We can also see the effect on chloride of one-time boosts
to magnesium when using magnesium chloride. Magnesium chloride
actually contains about three times as much chloride as magnesium,
so boosting magnesium gives an even larger boost to chloride.
Still, the natural chloride level is so high that the effects
are small on a percentage basis. Table 7 shows the effect
on chloride of several different boosts to the magnesium level.
That table clearly shows that boosting magnesium by even 300
ppm using magnesium chloride does not cause a substantial
swing in chloride. When corrected for the boost in salinity,
the effect is even smaller.
|
Table
7. Chloride Rise From a Single Magnesium Boost
Using Magnesium Chloride.
(Results are not corrected for salinity changes.) |
|
Magnesium
Boost (ppm) |
Starting
Chloride (ppm) |
Final
Chloride (ppm) |
Chloride
Rise (%) |
|
50 |
19,350 |
19,496 |
1
% |
|
100 |
19,350 |
19,642 |
1.5
% |
|
200 |
19,350 |
19,933 |
3
% |
|
300 |
19,350 |
20,225 |
5
% |
|
3. A certain mixture of magnesium
chloride and magnesium sulfate has no net effect on seawater's
major anions (chloride and sulfate). All that is necessary
for such a recipe is to add these two ingredients in such
a ratio that they add chloride and sulfate in the ratio naturally
present in seawater (which is 7.1 to 1 on a weight basis and
9.6 to 1 on a per ion basis).
To perfect such a recipe, it's imperative to know the amounts
of sulfate in Epsom salts (39%), the amount of chloride in
magnesium chloride hexahydrate (34.9%), and their bulk densities,
because most aquarists will use a volume based measurement
(1.05
g/cm3 for Epsom salts and 0.85
g/cm3 for magnesium chloride hexahydrate solids).
Taking all these factors into account, the desired volume
ratio is 10:1, MAG flake to Epsom salts, as a supplement;
for instance, 10 cups MAG flake and 1 cup Epsom salts.
Supplement Solutions
The easiest way to use these supplements
is to first make a solution in freshwater. Any of the three
different recipes may be chosen, but the second and third
are most useful for most aquarists.
1. Using Epsom salts only, dissolve 8 cups Epsom salts in
one gallon of water, and use that to supplement magnesium
in amounts determined by using this linked
online calculator, with the entry "Randy's Recipes
1 and 2 Versions A and B," and ignore for this purpose
what those designations mean. This recipe is the least preferred
of the three, but can be acceptable if used for small amounts
of supplementation, or if combined with at least 30% water
changes per month. It is also a more reasonable choice if
calcium chloride and sodium bicarbonate (baking soda) are
used in large amounts to supplement calcium and alkalinity.
2. Using MAG flake only, dissolve 8 cups magnesium chloride
hexahydrate in one gallon of water, and use that to supplement
magnesium in amounts determined using this linked
online calculator, with the entry "Randy's Recipes
1 and 2 Versions A and B," and ignore for this purpose
what those designations mean. This recipe is adequate, but
not quite as balanced as #3 below. This choice is not a good
way to go if calcium is supplemented by calcium chloride,
because together they will force chloride excessively high.
3. Using both Epsom salts and MAG flake, dissolve 7¼
cups MAG flake and ¾ cup Epsom salts in one gallon
of water, and use that to supplement magnesium in amounts
determined using this linked
online calculator, with the entry "Randy's Recipes
1 and 2 Versions A and B," and ignore for this purpose
what those designations mean. This recipe is preferred, but
its advantage over recipe #2 is minimal in most cases.
Note that combining the two materials in solution can result
in some precipitation of calcium sulfate (calcium and sulfate
are impurities in the MAG flake and the Epsom salts, respectively.
To assure yourself that the two materials have fully dissolved,
dissolve each separately in some freshwater before combining
them. Some calcium sulfate precipitation is acceptable, and
it is okay to let the solids get into the aquarium, assuming
you can dose in a way that prevents them from landing on delicate
organisms.
Note also that this recipe (#3) is different from that given
in my DIY two-part
recipe, because in that case more magnesium sulfate is
necessary to offset the rise in chloride that is provided
by both the calcium chloride and the magnesium chloride.
Using Magnesium Supplements
Whatever supplement you choose, I
suggest targeting the natural seawater concentration: 1285
ppm. When first using a DIY recipe, or even a commercial supplement,
add a much smaller dose the first time to be sure there is
no impurity (such as ammonia) that will negatively impact
corals. If you see negative effects, such as corals withdrawing
their polyps right after adding it, discontinue its use.
If you need to raise magnesium by several hundred ppm, splitting
the addition over several days will allow you to better home
in on the target concentration, and might allow the aquarium
to deal with impurities that come in with the supplement.
Conclusions
Magnesium is an important ion for
reef aquarists to understand and monitor. In addition to its
many biological functions, it serves to prevent the excessive
precipitation of calcium carbonate from aquarium water. Unfortunately,
magnesium often is depleted from reef aquaria as calcification
incorporates it into calcium carbonate skeletons and precipitates.
Because many ways of supplementing calcium and alkalinity
do not adequately supply magnesium to balance this consumption,
other means must be found. Commercial additives, such as those
from ESV and Kent, are adequate, but aquarists can also use
DIY materials that usually can be obtained at a lower cost.
Some of these recipes (and even some commercial supplements)
may skew the aquarium's ionic balance over time. Using Epsom
salts alone, for example, can cause sulfate to rise. While
the exact effects of elevated sulfate have not been clearly
established, it presents unnatural ionic chemistry to the
aquarium's inhabitants. A recipe using magnesium chloride
alone or, better yet, a combination of magnesium chloride
and Epsom salts, can produce a DIY recipe that is adequately
ionically balanced, easy to obtain and easy to use.
Happy Reefing!
|