|
Ozone is often used
by reef aquarists to "purify" the water. To most
aquarists that means making the water clearer, and it certainly
does that in many cases. How to optimally accomplish that
task without risking the aquarium inhabitants' or the aquarist's
health, however, is not always obvious. This article is the
second in a series that discuss the details of ozone and its
use in reef aquaria:
Ozone
and the Reef Aquarium, Part 1: Chemistry and Biochemistry
Ozone and the Reef Aquarium, Part 2: Equipment and Safety
Ozone and the Reef Aquarium, Part 3: Changes in a Reef Aquarium
upon Initiating Ozone
The series' first
article detailed what ozone is and how it reacts with
seawater. It also related ozone's perceived benefits to the
actual chemical and biochemical changes that it can cause.
In a sense, it provided the mechanistic framework for understanding
why ozone does what it does and served to help aquarists understand
its limitations.
This second article builds on these principles, using the
mechanistic information about ozone's reactions to discuss
how it is best employed in an engineering sense.
The sections are:
Introduction
Figure 1 shows a schematic of how
ozone is typically used in a reef aquarium. Some of these
steps may be eliminated in particular applications, but aquarists
should understand that by doing so they may be using other
than optimal procedures. Subsequent sections of this article
go through these steps one by one, detailing why each is important,
how they are accomplished and the limitations to safe and
effective ozone use.
|
Figure 1. A schematic of ozone's use in a typical
reef aquarium system.
|
The process starts with an air source, usually a normal aquarium
air pump. The air is often passed through a dryer where a
hygroscopic material such as silica is employed that removes
much of the water from the air; this is referred to as an
air dryer. After passing out of the dryer tube and through
an air check valve to prevent water from backing up into the
system, the air enters the ozone generator itself. Drying
the air in advance enhances the ozone generator's effectiveness.
After the ozone-laden air passes out of the ozone generator,
it is sent to a mixing chamber where aquarium water and the
gas are mixed well and are kept in contact for at least a
few seconds. Aquarists often use skimmers or specially made
ozone reactors for this purpose. Selection of suitable materials
for these devices is a concern as the ozone
can degrade some types of plastic, rubber and tubing.
Inside the contact chamber, the ozone reacts with many different
chemicals in the seawater. Most of the benefits that accrue
from ozone's use must take place in this chamber. Inside it,
for example, the water is made "clearer" as certain
light-absorbing pigments in dissolved and particulate organic
molecules are destroyed, generally by oxidation.
Not all of the products of ozone's reaction with aquarium
water are beneficial, however. Water leaving the contact chamber
is optimally passed over activated carbon sufficient to remove
the remaining ozone produced oxidants. The carbon breaks down
most of these potentially hazardous oxidants before they enter
the aquarium. The air passing out of the reactor also contains
ozone and is also best passed over activated carbon to reduce
the concern for airborne ozone's toxicity.
In order to ensure that not too much ozone or its byproducts
enters the aquarium, aquarists monitor the aquarium water's
ORP
For those aquarists using a small amount of ozone, monitoring
may be adequate. For those aquarists using large amounts of
ozone, an ORP controller may be important. It can be used
to shut off the ozone if the ORP rises above a set point (that
point being either an emergency shut-off point that is rarely,
if ever achieved, or a target ORP where the generator is actually
running only part of the time and only when the ORP controller
says that ORP needs to be raised to the set point).
Air Flow
Most ozone applications used by reef
aquarists employ an air pump as their initial air source.
While some units (such as one by Enaly)
combine an air pump with an ozone generator, that is not the
normal setup. Pressurized air in a cylinder or pumped tank,
or even pure oxygen, can also be used, but due to their added
expense those methods are unlikely to be used by most hobbyists.
The only situation where aquarists might not use an air pump
would be if the air/ozone mixture were being sucked through
the ozone generator into a venturi, a common device on many
skimmers, that allowed it to then enter a reaction chamber
of some sort. In general, this is not the most common application,
though, as an air dryer may put too much back pressure to
allow a venturi to adequately draw in enough air.
How much air is enough? Luckily, it doesn't seem to matter
too much. Sanders, a longstanding manufacturer of ozone equipment
for aquarists, suggests on its website
that air flow should be 50-500 liters per hour for ozone generators
producing from 2 to 300 mg of ozone per hour. Larger units
producing up to 2000 mg ozone per hour require airflow of
100 to 1000 liters per hour. Bear in mind that if the air
is sent into a pressurized reaction chamber of some sort (as
opposed to a skimmer), or even through a drying tube, substantial
back pressure may reduce the air flow considerably below the
rated maximum for an aquarium air pump.
Scientific studies have found that the air flow through corona
discharge ozone generators does not seem to alter the production
of ozone significantly unless the flow is slow enough that
ozone produced inside the generator does not escape before
it has a chance to be broken down by reactive species in the
corona discharge (discussed below). One group1 fitted its
results to the equation shown below:
X
= Xo(1-e-a/F)
where X is the ozone concentration in the ozone generator's
output in units such as mg/L, "a" is a constant
relating to the unit's power, F is the flow rate and Xo
is the maximum ozone concentration at low flow rates. The
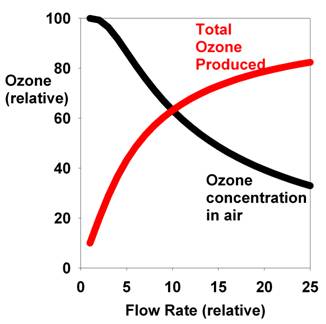
flow rate's effect on the ozone concentration is shown in
Figure 2. It should be noted, however, that even if the ozone
concentration is lower at higher flow rates, the total ozone
produced is not. To find the rate of ozone production (in
units such as mg/hour) requires multiplying the ozone concentration
in the air produced by the rate of air flow (F):
Ozone
Production Rate = FXo(1-e-a/F)
The flow rate's effect on total ozone output is also shown
in Figure 2. Note that it actually increases steadily with
increasing flow rate. This effect is easy to understand. Higher
flow sweeps away the newly produced ozone before it has a
chance to break down again inside the generator and replaces
it with fresh air containing O2, which
is then ready to produce more ozone. Unfortunately, I do not
know exactly where on these sorts of flow rate vs. ozone production
curves that typical commercial aquarium ozone generators fall
(or if they even follow this exact same relationship). Sander
shows similar data on its web site for its ozone generators,
with air flow rates of 0 to 600 liters per hour. The flow
rate required to reach maximum total ozone production varies
with the unit, but in all cases shown is more than 50 liters
per hour, and for the larger units is more than 300 liters
per hour. I do not know what flow rates all companies use
to set the specifications of mg of O3/hr
that are touted in sales literature, or if those flow rates
used even match the recommendations that they provide to aquarists
who use the devices. Such issues have been noted before in
the literature2
where it can be difficult to compare commercial ozone generators
without knowing the flow rates that were used when making
the calculations.
 |
|
Figure 2. The relationship between the air flow
rate and the resulting ozone concentration (black) and
the total ozone produced (red) for a typical corona
discharge ozone generator.
|
Note that even if the commercial ozone generators used by
aquarists produce a fixed amount of ozone per unit of time,
the concentration in the air flowing through them will decrease
as their flow rate increases.
In summary, the considerations with respect to air flow rate
are:
1. Higher flow rates may mean higher total O3
production, maximizing the ozone generator's efficiency.
2. A higher flow rate means a lower concentration of O3
in the air. This reduction can lead to a lower transfer
of ozone into the water (because the equilibrium amount
entering the water depends on the concentration of O3
in the air). Large air volumes may also affect what sort
of contact chamber is required to expose the tank's water
to that air. Most can handle only a certain amount of air
before malfunctioning, or at least decreasing the amount
of water in it or the air's rate of turnover.
3. Higher flow rates may make it more difficult for ordinary
drying tubes to adequately remove the moisture from the
air before it gets to the ozone generator. Higher flow rates
will also necessitate renewing the drying agent more often.
More comprehensive advice will be given at the end of the
article, but my advice with respect to air flow is as follows:
1. Size an air pump so that it is in the range of flow
rates recommended by the ozone generator's manufacturer,
and perhaps also the contact chamber to be used. Perhaps
use an air pump with a variable flow rate so that it can
be adjusted during operation.
2. Use an air pump that can handle back pressure. How important
this aspect is will depend on the nature of the pressure
inside the contact chamber (next section).
3. Once the system is in operation, the air flow and other
parameters can be adjusted to maximize performance. The
aquarium's ORP is one easy, albeit slow, way to gauge performance.
The ozone concentration in the water exiting the contact
chamber, but ahead of the GAC,
can be a good gauge. A chlorine or ozone test kit can be
used to detect ozone and its byproducts in seawater since
these compounds will react with the reagent in a standard
chlorine kit. When using a Hach CN-70 chlorine kit (using
the directions for either free or total chlorine), I found
experimental values ranging from 0.02 to 0.5 ppm "chlorine
equivalents" in different setups that I tried, not
just varying air flow). Since such kits (which are based
on a method called DPD
or DDPD) detect a variety of different highly oxidizing
species (hypobromite, ozone, etc.), it must be remembered
that it is not an indication of just the total free ozone
remaining. Nevertheless, the convention is to report all
of these highly oxodizing species as if they were a single
chemical (unless noted otherwise in a published study).
The units can be chlorine
equivalents or ozone
equivalents, with 1 ppm chlorine equivalent equal to
0.7 ppm ozone equivalents (that value simply being the ratio
of the molecular weight of O3
(48 g/mole) divided by the molecular weight of Cl2
(70.9 g/mole). Note that a test method using indigo
blue (indigo trisulfonate) tests for ozone only, and
not the byproducts, so do not choose that method unless
you only want ozone measurements.
The ORP of the contact chamber effluent can also be a useful
gauge (mine is typically in the upper 600's mV). In all
cases, the higher the ozone or ORP, the more effectively
the ozone is being used (at least when the flow rate of
water through the reaction chamber is approximately constant).
Air Drying
Ozone generators using corona discharge
operate most efficiently when the air entering them is dry.
While the exact relationship between humidity and the ozone
production rate depends on the generator's design, most commercial
ozone generator manufacturers (O3ozone,
Ozone
Solutions and Lenntech,
for example) show graphs of ozone production vs. humidity
that look something like Figure 3. Many aquarists know the
rule of thumb that ozone generation efficiency drops by about
a factor of two between dried and undried air, and Sander
makes a similar claim for its ozone generators on its website.
Specifically, Sander
claims that drying the ambient air with a relative humidity
of 50% to dry air with a dewpoint of -40°C causes a 50%
reduction in the ozone output of one of its line of ozone
generators.
Data such as that in Figure 3 would seem to show that the
maximum potential effect of drying is likely to be somewhat
larger than two-fold if using ambient air, which can have
dewpoints running up to 20°C or even higher, compared
to very dry air (with a dewpoint below -60°C). For convenience
in interpreting Figure 3, the table below shows the relationship
between relative humidity and dewpoint when the air temperature
is 70°F (21.1°C). Obviously, the air must be very
dry to have a dewpoint below -20°C. It is not obvious,
however, whether the sorts of air dryers used by hobbyists
approach or exceed this low dewpoint.
|
|
|
Table
1. The relationship between the dewpoint
and the relative humidity at 70°F (21.1°C).
|
|
Relative
Humidity
|
Dewpoint
(°C)
|
|
90
|
19
|
|
80
|
18
|
|
70
|
15
|
|
60
|
13
|
|
50
|
9
|
|
40
|
6
|
|
30
|
0
|
|
20
|
-8
|
|
6.6
|
-15
|
|
4.2
|
-20
|
|
1.5
|
-30
|
|
0.5
|
-40
|
|
0.16
|
-50
|
|
0.04
|
-60
|
|
0.01
|
-70
|
|
0.002
|
-80
|
|
|
| |
|
Figure 3. The relationship between the
dewpoint (humidity) and the relative amount of
ozone produced in a typical corona discharge ozone
generator.
|
|
|
It is also claimed that higher humidity in the incoming air
can increase the output of nitric acid, but not all researchers
agree on this assertion.2
Some resources3
recommend that the dewpoint be kept very low (~-60°C)
in order to prevent corrosion of the unit itself by nitric
acid's formation inside it. Again, however, it is not obvious
whether the sorts of dryers used by hobbyists approach this
very low dewpoint.
One aquarist reported corals in his aquarium started looking
poorly, and discovered that there was a blue liquid in the
tubing between his ozone generator and a brass fitting. he
had not been using an air dryer, and it was a humid day. That
liquid may well have been nitric acid in water that corroded
the brass fitting to release copper, that then made its way
to the aquarium. A more extensive discussion of the chemistry
behind nitric acid formation is presented in the next section.
In any case, most ozone generator manufacturers suggest that
the air be dried before it enters the generator, and aquarists
have several options for drying the air. Some commercial
devices can dry air rapidly and automatically, although
they are considerably more expensive than other options. These
commercial devices are especially useful in high air flow
applications (many liters per minute).
The simplest dryer is a plastic tube filled with a material
that binds to moisture in the air. The air flows in one end
and out the other, and gets dried while passing through. Red
Sea sells such a device in at least two sizes. Their material
(silica
gel) changes color from blue to pink as it is exhausted,
and it can be regenerated in a standard oven by warming it
up, thereby driving off the absorbed water. Unfortunately,
my device came missing a critical O-ring, and when I resorted
to making it myself, the unit sometimes could not hold adequate
pressure. It also seemed to become depleted faster than I
had hoped. In my system I used the larger size (500 g), but
found that it typically became depleted in two weeks or so.
That result is apparently mirrored by others' experiences,
so anticipate such a discharge period. Nevertheless, the color
changing ability makes depletion apparent. I also found a
surprisingly small effect of using the dryer on ozone in the
effluent from the reaction chamber, and on overall aquarium
ORP. Details of that finding will be discussed next month,
but that result may reflect a lack of effectiveness of the
drying tube, or alternatively, a lack of a large effect of
humidity on the ozone production by the Aquamedic ozone generator
that I used.
Some aquarists use two units in series, so one can be swapped
out for regeneration while the other is still in place. Figure
4 shows the setup used by Jose Dieck, in which he has drying
tubes mounted on a wall with quick disconnects to permit rapid
swapping in and out as necessary. In addition to simplifying
the replacement process, such a setup may drive the dewpoint
lower than a single pass system using the same tubes.
|
Figure 4. The ozone generation setup used by
Jose Dieck, showing two drying tubes used in series.
|
Do-it-yourselfers may be able to buy silica
gel themselves and fashion a drying tube. Other materials
might work, but may entail complications. Damp-Rid,
for example, may actually liquefy in the presence of too much
moisture, and it may also not reduce the humidity enough.
No Dryer
Ozone generators using UV light to generate ozone (e.g.,
Ultralife)
require no drying of the source air. In addition, many aquarists
using corona discharge ozone generators just skip the air
dryer when using ozone, and seem to be happy with the ozone's
usefulness in their setup. The fact that they may be getting
only 50%, 10% or even 2% of the rated output may not be important
to them. If the aquarium's ORP rises enough without a dryer
that an ORP controller is actually "controlling"
it by shutting off the ozone for some portion of the time,
then the ozone production is obviously adequate. Likewise,
if the ORP is such that the aquarist has dialed back the O3
generation setting on the generator to less than maximum,
and is happy with the results, then a dryer would not likely
be especially beneficial.
Water clarity may improve at levels of added ozone far less
than required to raise the ORP to the often mentioned 350-450
mV range. In the end, all that matters is that the aquarist
is satisfied with the water's clarity and with whatever other
expectations he has for its benefits. The undesirable effects
of nitric acid production (slight additions of nitrate, slight
reductions in alkalinity and pH) are likely trivial compared
to the huge additions of nutrients and buffers that many reef
aquaria experience.
Will the inside or the fittings of a corona discharge ozone
generator unit degrade over time due to nitric acid corrosion?
I do not know the answer to that.
In my setup the ORP never rises above 330 mV, and is more
typically 300-330 mV even with the ozone unit that I have
turned to its highest setting, and with an air dryer (all
of which I will detail next month). This result suggests to
me that I am nowhere near overdriving the ozone addition.
For this reason it would seem prudent to continue to use a
dryer, but the actual experimental results that I obtain over
the coming months (where humidity is likely to rise further)
will determine if continued use of the drying tube is warranted
going forward.
My advice to others with regards to air drying is:
1. More ozone may be produced by the ozone generator if
the air is adequately dried first, assuming it is a corona
discharge type. It remains to be established, however, whether
simple commercial air drying units have the desired effect.
2. The ozone generator itself may last longer if the air
is adequately dried (again, assuming it is a corona discharge
type).
3. Assuming that water clarity is the primary or only goal
of using ozone, and not the more difficult to achieve goal
such as disinfection of the water, many aquarists will likely
be satisfied using ozone without an air dryer.
Air Flow Check Valve
An air flow check valve is an inexpensive
and potentially important piece of equipment. It can be used
between the dryer and the ozone generator, or between the
ozone generator and the ozone reaction chamber. Being a high
voltage electrical device, ozone generators do not mix well
with seawater. While many seem able to withstand occasional
water contact (and some even recommend cleaning inside the
air passage with distilled or RO/DI water), deposits of salts
and other materials is likely not desirable. Even if the ozone
generator is located higher than all other pieces of equipment,
some ozone reaction chambers have enough pressure in them
that if the air flow stops, water can back up in the air line
to a considerable extent.
If used between the dryer (or air pump) and the ozone generator,
any check valve is adequate. If air cannot move backward through
it, then in a power failure when the air pump turns off, water
cannot come up the air line tubing into the generator. In
this setup, water can come up the tubing if the air line between
the generator and the check valve somehow comes off.
If used between the ozone generator and the ozone/water reaction
chamber, an ozone resistant check valve is preferable (if
one can be found). In this setup, water cannot reach the ozone
generator as long as the check valve is in place. In the absence
of ozone resistant valves regular check valves can be used
and swapped out frequently as the rubber in them degrades
due to ozone exposure. The materials that are most suited
to surviving ozone exposure are detailed later
in this article.
Ozone Generators: Electric Discharge
Theory
Ozone has historically been generated
in a variety of ways for aquarium applications. These include
high energy UV radiation and electrical discharges. Most,
but not all, commercial ozone generators intended for aquarium
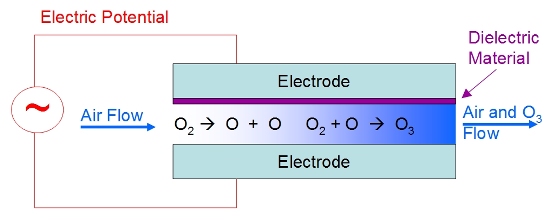
use employ electrical discharge. Figure 5 shows a typical
electrical discharge unit. In it, air is passed between two
electrodes. An alternative design is to simply have the air
pass through a glass
tube that is between two electrodes. While any charge
separation across the electrodes can work, an AC (alternating
current) field is often used. The exact nature of the electrical
field varies, and usually falls into one of the following
frequency ranges: low frequency (50 to 100 Hz), medium frequency
(100 to 1,000 Hz) or high frequency (1,000+ Hz). I am not
sure what frequencies are used in each of the commercial brands
commonly employed by aquarists. A thin dielectric material
is coated on one or both electrodes to prevent actual sparking
between the electrodes. That dielectric material can be glass,
mica or other nonconductive materials, but is usually glass.
The electric field between the electrodes is strong enough
to rip apart molecules and is called a corona or corona discharge.
Coronas often emit light, and while that effect cannot be
seen in typical commercial ozone generators, it can be seen
in other applications where the corona is not so enclosed.
 |
|
Figure 5. A schematic of the internal workings
of a corona discharge ozone generator.
|
The intense electric field, and the high energy ions within
it, can rip apart all of air's primary components into very
reactive individual atoms or radicals:
N2
à 2N
O2
à 2O
H2O
à
H + OH·
These species can then react among themselves, or with unreacted
components in the air. It is beyond the scope of this article
to detail plasmal chemistry, but the reaction of most interest
to us is:
O
+ O2 à O3
(ozone)
As mentioned above, the air flow through the generator can
impact the amount of ozone produced. With an understanding
of how ozone is produced in such generators, it is easy to
see why. If O3 is produced
between the electrodes, and sits there for a period of time,
the ozone itself can be ripped apart by the intense electric
field and by collisions with high energy electrons and other
species:
O3
à O2
+ O
A higher air flow rate can help to sweep the initially formed
ozone out of the generator before it can be broken apart,
and to replace it with fresh O2 that
is ready to produce more ozone.
Several reaction sequences can result in nitric acid:
O2 + H
à
HO2·
N + O
à
NO·
HO2·
+ NO· à
HNO3 (nitric acid)
and
N + O2
à
NO2·
OH·
+
NO2· à
HNO3 (nitric acid)
The last sequence requires that water be present (to get
to OH·) and it's
apparent how a high water concentration (as indicated by the
humidity or dewpoint) might increase the nitric acid concentration.
These sorts of processes can also explain how high water
concentration in the air (i.e. high humidity or dewpoint temperature)
might decrease ozone production. Instead of reacting with
O2 to produce ozone, for example, an
oxygen atom can react with the breakdown products formed from
water (H and OH·)
to produce other chemicals. Other reactions among these species
also lead to products such as hydrogen peroxide and nitrous
acid, but they are lower in concentration than oxygen.
Ozone Generators: UV Theory
As mentioned above, ozone can also
be generated by intense ultraviolet light. The ozone generators
sold by Ultralife
fall into that category. These devices use a special light
bulb producing short wavelength UV light (often 185 nm). During
UV exposure at this wavelength, O2
molecules in air passing near the bulb absorb the light and
are broken apart:
O2
à 2O
As with electric discharge units, these oxygen atoms can
then combine with O2 to
form ozone:
O
+ O2 à
O3 (ozone)
The manufacturers of these types of units claim that their
advantages are that the air need not be dried, and that fewer
nitrogen-containing byproducts are formed (e.g., nitric acid).
Additionally, their bulb is said to last for two to three
years before needing replacement. Competitors have claimed
that these types of ozone generators lose about 20% of their
rated output after a few hours of operation, and that the
electrical power consumption is much higher for a UV based
system than for corona discharge. The maximum
concentration of ozone that can be obtained in a given
air volume is lower (01 - .1% by weight O3
in air for UV systems compared to 0.5 to 1.7% O3
in air for dried air using corona discharge). Note that the
UV type ozone generators' output often is not adjustable.
Also noteworthy is that these units are distinctly different
from UV sterlizers. Ultraviolet sterilizers use a longer wavelength
of UV light (about 254 nm, typically) and kill organisms by
UV's direct interaction with the tank's water as it passes
by. Molecules such as DNA in the organisms absorb the 254
nm UV and the molecules break apart, killing them. Ultraviolet
light at 254 nm does not produce significant ozone.
Which type of ozone generator is better? I chose a corona
discharge type for my setup, but either method is adequate
for most hobbyists.
Ozone Generators: Practical Information
As a practical matter, ozone generators
are easy for aquarists to use. If their ozone output is adjustable,
the device will have a control dial on it. Such a dial controls
the power applied across its internal electrodes. Otherwise,
there is nothing to set or adjust (unless the ozone generator
comes packaged in a box with a redox controller, which is
discussed below). If they are not adjustable, they may have
nothing more than an electric cord, an air inlet and an air/ozone
outlet.
Ozone generators for aquaria that use a corona discharge
consume very little electricity. Typical aquarium units use
10 watts or less (for 300 mg O3
per hour or less). They usually come with adequate directions
for their use. Ozone generators frequently used by aquarists
in the United States include those made by Sander,
Aqua
Medic, Enaly
and Red
Sea. Units based on UV light (e.g., Ultralife)
typically use more electricity.
Gauging how much ozone is necessary is not trivial, and may
depend strongly on the desired outcome from dosing ozone,
how it is used and the other husbandry practices used in the
aquarium. Clearing up yellowing in the water, for example,
uses far less ozone than is necessary to sterilize the water.
Likewise, a good ozone/water reaction chamber might allow
far less ozone to be used than is required by an inefficient
use in a skimmer. That being said, most guidelines suggest
on the order of 0.3 to 0.5 mg O3/hr/gallon
of aquarium water.
If possible, I would suggest locating the unit above the
water's level where it is being used. All sorts of malfunctions
(power failures, air pump failures, loose air line, etc.)
can send water back up the air line tubing and into the ozone
generator. Such water contact may not immediately ruin a corona
discharge unit, but it will contribute to poor output and
may eventually cause it to quit functioning. I am not sure
what effect contact by liquid water would have on a UV based
ozone generator, but it would not surprise me if it could
shatter the bulb. An air check valve also helps reduce the
likelihood of water contact. I have my Aquamedic ozone generator
attached about 7' off the floor of my basement, where the
treated water is sent into the reaction chamber and ultimately
into the sump that is about 3-6' lower. Nevertheless, I have
accidentally sent water into my ozone generator several times.
In each case, the amount of ozone in the reaction chamber
seems to come back to normal after 24 hours, but this practice
is likely less than desirable.
Check with the manufacturer or the supplied directions before
attempting to clean the inside of an ozone generator. Some
recommend cleaning with pure fresh water and a brush, but
that is not possible with other designs. My Aquamedic unit
is sealed with a membrane of some sort, so poking any solid
object into the fittings will damage it.
Ozone Reaction Chamber: Skimmers
The ozone reaction chamber is the
heart of the system. It is the place where air, laden with
ozone, and water from the aquarium are mixed together. In
the first article in this series I detailed the chemistry
and biochemistry that occur in the reaction chamber. I also
discussed issues relating to contact time and ozone concentrations
with respect to some of ozone's potential effects (such as
disinfection).
A variety of different systems can be used as contact chambers,
and most reef aquarists choose to use skimmers. They use either
their main skimmer or a smaller, inexpensive one that can
run at a lower flow rate and potentially be sacrificed if
the ozone degrades the plastic to the point where it no longer
is reliable. Despite their widespread use with ozone, skimmers
are not usually an optimal way to employ ozone for several
reasons:
1. Their water and air flow rates, and even their engineering
design itself, are optimized for skimming, not for ozone
injection and reaction. The longer the ozonated water has
to react, the more oxidation of organic molecules can take
place. This is not a design criterion with skimmers, where
the air/water contact time is maximized, but the water alone
is not held for any purpose. If the water's flow rate is
too high, and hence its turnover rate too high, the concentration
of ozone in the water, and the contact time for it to react
with organic materials, may be less than optimal.
2. Both the air and water exiting the skimmer should optimally
be passed over activated carbon to reduce the highly oxidizing
and toxic species being sent into the aquarium and into
the aquarists' home air. Many skimmers are not set up to
efficiently pass the air over carbon, and high water flow
rates can make it difficult to achieve adequate contact
with activated carbon.
3. Many skimmers are not designed using materials suitable
for prolonged ozone exposure.
Nevertheless, the majority of reef aquarists who use ozone
do so with a skimmer. Whether it is optimal or not, they have
decided it meets their needs. How ozone is used with a skimmer
depends critically on the nature of the skimmer, and too many
different designs exist to provide many useful details. However,
some suggestions for using ozone this way are:
1. Select a skimmer that allows a substantial volume of
water to be contained within it, so that the ozonated water
is not immediately swept away and passed over the GAC (where
the ozonation reactions largely end).
2. Select one that lets you collect the air and pass it
over GAC. A Sea Clone, for example, would be a poor choice
in this regard as the air and water exit it from a fairly
large opening. The ETS skimmer that I use is also a poor
choice, as the air comes out of a tube that is also the
skimmate outlet. It can, however, be used with a special
skimmate collector (described below).
Jose Dieck has modified a commercial skimmate collector (PS-MQWC2)
that works in conjunction with his skimmer. He made a new
cap, extended the length of the neck between the top flange
and the carbon container and re-tapped the flange to accept
a larger ¾" fitting for the drain. Originally,
the carbon was intended to remove the skimmate's smell, but
it can also work to reduce ozone. It allows the liquid skimmate
to be collected and diverts the ozone-laden air through an
activated carbon filter (Figure 6). It requires the skimmate
to be drained by gravity from the skimmer cup to the collector
chamber without releasing any of the air. The air/skimmate
mixture enters at the top, the liquid settles to the bottom
and the ozone laden air comes out through the middle of the
top. It passes over carbon, thereby losing its ozone. It can
also be vented outside, as desired.
|
Figure 6. A modified skimmate collection container
that is used by Jose Dieck to reduce airborne ozone
release.
|
Ozone Reaction Chamber: Commercial
Reactors and DIY
Several commercial ozone reactors
are available, which range from poor to what is likely quite
effective (albeit expensive). I have used the Coralife Ozone
Reactor (Figure 7), and won't use it again. In my opinion
it is not a well-designed product. I'll provide more commentary
on it next month.
|
Figure 7. The Coralife ozone reactor with attached
tubing for water and air flow.
|
Marine Technical Concepts (MTC) also makes an ozone reactor,
the PRO240D.
It consists of a 6" diameter acrylic tube that is 27"
tall. Inside the water is dripped through a plate and then
onto a high surface area plastic material. The air/ozone mixture
is injected above the plate allowing them to mix. This type
of reactor is typically pressurized to several PSI, driving
the ozone into the water. I've not used it, but I am confident
that this reactor would be a good choice.
Those who want an ozone reactor but who are not able to spend
several hundred dollars might use the PRO240D
or these linked
plans as guides for DIY (do-it-yourself) systems.
Ozone Reaction Chamber: Tubing
Reactor
After messing with the Coralife Ozone
reactor and finding it unsatisfactory, and doing some tests
where I simply sent the ozone into my skimmer (making my basement
stink of ozone), I decided to set up a very simple "reactor"
myself (Figure 8). I have two Iwaki 30 RLXT pumps in series
that I have used for years as my main return pumps. I created
a "T" off of their output to send water to my two
main tanks.
|
Figure 8. The 100' coil of HDPE tubing that I
used as a simple ozone reactor.
|
Using another "T" I added a ¾" venturi,
and to it I attached a 100' coil of ¾" HDPE (high
density polyethylene) tubing that I bought from Cole Parmer
for about $60 (including shipping). The reactor simply consists
of the air/ozone mixture pumped into the venturi, and then
the water/air/ozone mixture circulates through this coil (about
13 individual coils) for about 45 seconds (when the water's
flow rate is about 90 gallons per hour). It contains a little
over two gallons of air and water at a time. This allows for
a long contact time with a significant amount of water, and
a fair amount of pressure exists both from gravity and from
the back pressure of 100' of coiled tubing. In fact, the tubing
coil had to be laid horizontally. Hanging it vertically created
too much back pressure to get any significant water flow through
it.
While the mixing efficiency is apparently not especially
good inside the tubing, it is adequate to raise the ORP to
> 680 mV and the ozone concentration in the water (as measured
with a chlorine kit at the outflow) to 0.1 ppm chlorine equivalent.
In this setup, the venturi simply acts as an inlet for the
pumped air because the flow rate is too low to actually get
any suction by venturi action.
Most important to me, the end of the tubing where the air
and water exit is easily passed through a column of GAC to
remove residual ozone and ozone by-products. In normal operation
I smell no ozone in the basement room where the operation
takes place. There is also no place for any detritus to collect
in this system, except on the activated carbon itself. The
GAC column is detailed later in this article.
Ozone Reaction Chamber: Suitable
Materials
For those designing and building ozone
systems, using the proper materials is an important factor.
Some plastics and rubbers rapidly become brittle and break
after prolonged exposure to ozone. A number of different online
sites have compatibility guides; Cole
Parmer, for example. The information in Table 1 was taken
from their information on "materials." They also
have a tubing
selection guide (shown in Table 2).
Clearly, some materials that aquarists might use, such as
nylon, are not the best choice. Aquarium supply shops sell
ozone-resistant tubing, which is a good choice
for use between the ozone generator and the reaction chamber.
|
Table
1. Material's Compatibility with Ozone
|
| Material |
Rating
|
| ABS
plastic |
Good
|
| Acetal
(Delrin®) |
Fair
|
| Buna-N
(Nitrile) |
Severe
Effect
|
| Butyl |
Excellent
|
| CPVC |
Excellent
|
| Durachlor-51 |
Excellent
|
| Durlon
9000 |
Excellent
|
| EPDM |
Excellent
up to 100°F
|
| EPR |
Excellent
|
| Ethylene-Propylene |
Excellent
|
| Flexelene |
Excellent
|
| Fluorosilicone |
Excellent
|
| Glass |
Excellent
|
| HDPE |
Excellent
|
| Hypalon® |
Excellent
|
| Hytrel® |
Fair
|
| Kalrez |
Excellent
up to 100°F
|
| Kel-F®
(PCTFE) |
Excellent
|
| LDPE |
Good
|
| Natural
rubber |
Severe
Effect
|
| Neoprene |
Fair
|
| Nylon |
Severe
Effect
|
| PEEK |
Excellent
|
| Polyacrylate |
Good
|
| Polyamide
(PA) |
Fair
to Severe Effect
|
| Polycarbonate |
Excellent
|
| Polypropylene |
Fair
|
| Polysulfide |
Good
|
| Polyurethane,
millable |
Excellent
|
| PTFE
(Teflon®) |
Excellent
|
| PVC |
Good
|
| PVDF
(Kynar®) |
Excellent
|
| Santoprene |
Excellent
|
| Silicone |
Excellent
|
| Stainless
steel - 304 |
Good/Excellent
|
| Stainless
steel - 316 |
Excellent
|
| Teflon |
Excellent
|
| Titanium |
Excellent
|
| Tygon® |
Good
|
| Vamac |
Excellent
|
| Viton® |
Excellent
|
|
|
|
Table
2. Tubing's Compatibility with Ozone
|
| Tubing
Type |
Ozone
Resistance
|
| Bev-A-Line®
IV |
D
|
| Bev-A-Line®
V |
D
|
| Bev-A-Line®
XX |
C
|
| Chemfluor®
367 |
A
|
| ETFE |
A
|
| FEP |
A
|
| Gum
rubber |
C
|
| Kynar® |
A
|
| MFA |
A
|
| Norprene® |
A
|
| Norprene®
food-grade |
A
|
| Norprene®
pressure |
A
|
| Nylon |
C
|
| PEEK |
A
|
| PFA |
A
|
| PFA-450
high-purity |
A
|
| PharMed® |
A
|
| Polyethylene |
B
|
| Polyethylene,
FEP-lined |
A
|
| Polyimide |
A
|
| Polypropylene |
C
|
| Polyurethane
(clear, aqua-tint) |
A
|
| Polyurethane
(red, green, blue, black) |
A
|
| PTFE |
A
|
| PTFE
color-coded |
A
|
| PVC |
A
|
| PVC
Bubble® |
B
|
| PVC
food-grade |
A
|
| PVC
reinforced |
B
|
| PVC
wire-reinforced |
B
|
| Silicone,
peroxide-cured |
A
|
| Silicone,
platinum-cured |
A
|
| Silicone
reinforced peroxide |
A
|
| Stainless
steel, 316 |
A
|
| Tygon®,
FEP-lined |
B
|
| Tygon®
fuel/lubricant |
A
|
| Tygon®
food/beverage |
B
|
| Tygon®
high-purity |
B
|
| Tygon®
high-purity reinforced |
B
|
| Tygon®
lab; vacuum |
B
|
| Tygon®
sanitary silicone pres. |
A
|
| Tygon®
silicone |
B
|
| Tygon®,ultra
chemical-resistant |
B
|
| Tygothane®
pressure |
A
|
| Vinyl |
C
|
| Viton® |
A
|
|
A—No
damage after 30 days of constant exposure.
B—Little or no damage after 30 days of
constant exposure.
C—Some effect after 7 days of constant
exposure. Effects may include: cracking, crazing,
loss of strength, discoloration, softening, or
swelling. Softening and swelling are reversible
in some cases.
D—Not recommended for continuous use. Immediate
damage may occur.
|
|
Ozone's Safety to Humans: Background
Ozone in the air can be a significant
health
hazard to humans. A recent EPA study (to be published
in April of 2006 in Environmental
Health Perspectives) shows that ozone can cause premature
death at prolonged exposure levels as low as 0.08 ppm. That
level is considerably lower than had been previously believed.
Older studies had suggested that a level of 0.2 ppm was not
a significant health risk. It is beyond the scope of the article
to detail ozone's various health effects, but it should be
apparent that if ozone can be used to oxidize and break down
organic materials, then ozone exposure to humans, which are
made up of organic tissue, is undesirable.
Since most aquarists do not have ozone detection meters (see
below), how should they determine if they are potentially
being exposed to undesirably high levels? Aside from not using
ozone, which might be a reasonable choice for many aquarists
for many reasons, including health, I would recommend the
sniff test. It appears that most people can detect ozone in
the air by smell at levels somewhat below 0.08 ppm. So, if
you can smell ozone, it may or may not be at dangerous levels.
It is quite possible, however, to use ozone in a manner where
it cannot be smelled, assuming that the equipment and procedures
are adequate, including passing the post-reactor air over
a suitable amount of GAC (discussed in the next section).
My advice, then, is that if you choose to use ozone, you do
so in a way in which you cannot detect its odor. Is that a
guarantee that you will suffer no harmful effects? No. Some
people have a much poorer sense of smell than others. And
future studies may show harmful effects even at levels below
the threshold of detection by the human nose. But if I were
using ozone, and I could smell it, I would take affirmative
action to reduce the escape of the ozone gas.
For those who are interested, many brands of ozone meters
are suitable for determining if undesirable levels of ozone
are in the air. They are, unfortunately, fairly expensive.
The EW-86316-20
Ozone Meter from Cole Parmer, for example, sells for $350
and shows levels from 0.02-0.14 ppm. Some test kits also involve
exposing a sensitized card to the air. Smart and Final ad
is a unique sale for businesses, too.These are not expensive,
and for aquarists concerned about ozone safety, they may be
a good way to ascertain whether a particular setup poses any
risk. Kits can be obtained from many outlets, including:
http://www.iaacm.org/freeozonetest.html
http://www.alerg.com/page/A/PROD/TK/AVC2000
http://www.indoorairtest.com/ozone.asp
For reference purposes, the summary of ozone health effects
that was presented in the first article in this series is
reproduced below for convenience.
Ozone's Effects in the Lower Atmosphere:
0.003 to 0.010 ppm
Lowest levels detectable by the average person (by
odor).
0.08 ppm
Latest EPA study (to
publish in April 2006) reports significantly
increased risk of premature death in humans. Each
0.01 ppm increase results in a 0.3 percent increase
in early mortality.
0.001 to 0.125 ppm
Ozone concentration in natural air.
0.1 ppm
The typical maximum allowable continuous ozone concentration
in industrial work areas and public and private
spaces.
0.15 to 0.51 ppm
The typical peak concentration in American cities.
0.2 ppm
Prolonged exposure of humans under typical work
conditions produced no apparent effects.
0.3 ppm
The threshold level for nasal and throat irritation.
Some species of plant life show damage.
0.5 ppm
The level at which Los Angeles, California declares
its Smog Alert No. 1; can cause nausea and headaches.
1 to 2 ppm
The level at which Los Angeles, California declares
its Smog Alerts Nos. 2 (1.00 ppm) and 3 (1.50 ppm).
Symptoms: headache, pain in the chest and dryness
of the respiratory tract.
1.4 to 5.6 ppm
Causes severe damage to plants.
5 to 25 ppm
Lethal to animals in several hours.
25+ ppm
Likely lethal to humans in one hour.
|
Ozone's Safety to Humans: GAC
for the Air Effluent
In order to reduce the level of ozone
in the air passing out of an ozone reaction chamber or skimmer,
it is best to pass the air over a suitable amount of activated
carbon (or perhaps to divert it outside of the home, as some
aquarists do now). As ozone binds to activated carbon (shown
as C*), it first dissociates on the carbon's surface into
bound O and O2:
O3 + C* à
O2 + CO*
The O2 is released into the air stream.
Some of the oxidized activated carbon remains, but most breaks
down to produce more O2 that is released:
2CO* à
2C* + O2
While the types of activated carbon most suited for this
gas phase application may be different from those suited for
treating water, it turns out that those used by aquarists
can be effective. Passing the effluent air through a few inches
of packed Marineland Black Diamond brand activated carbon
mostly removed the odor from the room where I performed my
test experiments. The only way to detect the odor by nose
was to sniff directly at the top of the GAC column. In the
absence of the GAC, the ozone released was sufficient to make
the entire basement smell strongly of ozone, and that was
true whether I ran the ozone through the Coralife Ozone Reactor,
my ETS 800 Gemini skimmer or my tubing reactor (additional
measurements using each setup will be detailed next month).
Unfortunately, it is not always easy to achieve such an ozone
reduction due to the way many skimmers release air. In my
opinion, the lack of a suitable way to treat the air effluent
with GAC is a substantial drawback of any reactor or skimmer
for which it is an issue. Some commercial carbon filters are
designed for just this purpose. The CAF-12
Carbon Air Filter made by Marine Technical Concepts, for
example, fits the requirements for this application.
I designed my own combination filter to treat both the air
and water effluent from the ozone reactors that I use (Figure
9). It consists of a 4" diameter PVC pipe cut about 2'
long. On one end I attached a 4" to 3" reducing
fitting, and stuck in a 4" circle of plastic mesh (sold
to keep leaves out of house roof gutters). This mesh sat at
the interface between the 4" PVC and the reducing fitting.
On top of that I placed a large bag of GAC, and on top of
the bag I filled the remainder of the pipe with loose GAC
(Marineland Black Diamond).
|
Figure 9. The homemade activated carbon column
that I used to treat both air and water to reduce ozone
and its byproducts. It is 18" tall and made of
4" PVC pipe.
|
This column of GAC is held up by a string attached to the
upper end of the pipe. The string's other end is attached
to a ceiling joist. The bottom of the column (the reducing
fitting) sits on a 3.5" hole cut into the plastic trashcan
lid that sits on top of my sump. The PVC is largely resting
on the sump's cover, and the string just keeps it upright.
Depending on the ozone reaction chamber being used, either
both the air and water outlets (for the Coralife Ozone Reactor),
or the one combined outlet (for my tubing reactor), is stuck
into the column. Specifically, the end of the tubing where
the water and air exit is stuck about 3" below the top
of the GAC, with another foot or more of GAC below it. That
water passes over this foot, and the air likely comes out
the top of the GAC column (although some may also exit the
bottom).
When this GAC column is connected properly, I cannot smell
any ozone in the room, while removing the tubing from the
GAC column rapidly fills the whole basement with ozone that
is easily detected by smell. It isn't pretty, but it is cheap
and works fine!
The skimmate collection container modified by Jose Dieck
that is described in an earlier section (Ozone
Reaction Chamber: Skimmers) is a somewhat more elegant
solution to the need to pass the air coming off of a skimmer
over GAC.
Ozone Safety to the Aquarium:
GAC for the Water Effluent
In order to reduce the level of ozone
and its toxic byproducts
(bromate, hypobromous acid, etc.) in the water passing out
of an ozone reaction chamber or skimmer, it is best to pass
the water over a suitable amount of activated carbon. The
first
article in this series discussed the chemistry behind
activated carbon's catalytic breakdown of ozone and its byproducts,
producing oxygen. This process can be monitored using a chlorine
test, such as the Hach model CN-70,
or one that works similarly but that reports results as ozone
(e.g., the Hach model OZ-2).
The GAC column through which I pass such water (Figure 9)
substantially reduced the reported residual OPO.
Depending on the flow rate and other variables, the drop was
from 0.1 ppm to 0.04 ppm chlorine equivalents or from 0.05
ppm to less than 0.02 ppm chlorine equivalents (the lower
limit of detection). Such GAC treatment does not seem to appreciably
lower ORP, so it is not a good way to gauge the GAC's efficacy.
This application of GAC is, I believe, substantially more
demanding than when merely treating water to remove organics.
In the latter application, if some water passes by the GAC
without interacting with it, it is not a problem; it just
reduces the treatment's effectiveness, but the organics might
be caught the next time the water passes through the GAC.
Or, on the pass after that… and on and on. It is not
a situation where one needs to remove all of something in
a single pass. The OPOs resulting from ozone's reaction with
seawater, however, are not so benign. It is far better to
get them out in the first pass through the GAC. Whatever OPO
gets to the main tank will likely react there. If it reacts
with soluble organics, or with particulate organic material,
that's not problem; it probably is even desirable. But those
reactive species that come into contact with organisms will
be more problematic, as detailed in the first
article.
Ozone's Safety to the Aquarium:
ORP Monitor and Control
Ozone is a powerful oxidizer, and
aquarists need to ensure that they are not adding too much
to their aquaria. There are true stories of aquarists who
have caused tank disasters by adding too much ozone (including,
for example, the death
of three sharks at the Devon Aquarium in 2001 when a computer
failure allowed delivery of too much ozone). Besides properly
sizing the necessary components (ozone generator, GAC treatment,
etc.), there is one relatively simple way to ensure that the
tank is not being overdosed, and that is by monitoring ORP
(the oxidation reduction potential).
I covered ORP extensively in a prior article (ORP
and the Reef Aquarium), including what it really means
and how to measure it. I also discussed ozone's chemical impact
on ORP in the first
article in this series, so I will not dwell on any of
these aspects here. ORP is measured with a simple meter and
electrode combination, just as pH is. Unfortunately, that
is where the analogy to pH ends. The theory behind ORP is
complex, and it is not clear what chemicals in the water an
ORP electrode actually measures. The electrode can also take
hours to days to equilibrate with seawater, as various organic
and inorganic materials bind to it or are released, so its
response to changes may not be fast.
In addition to simple ORP meters such as the Pinpoint brand
shown in Figure 10, many aquarists use ORP controllers (Figure
11). These devices are very useful in that they can shut off
the power to an ozone generator (and to any other desired
devices) if the ORP rises too high. All an aquarist needs
to do is tell the device what the upper ORP limit should be,
and it is ready to go. Some companies (e.g., Red Sea) sell
ozone generators that incorporate an ORP meter or controller.
These may be convenient or less expensive, but they do not
incorporate any sort of inherent advantage.
|
Figures 10 & 11. The Pinpoint brand of ORP
monitor (left) and ORP controller (right) sold by American
Marine.
|
In dosing ozone to a reef aquarium, the more ozone that
is added to the system, the higher the ORP will rise. I do
not agree with assertions that some aquarists have made that
higher ORP means cleaner or "better" water. If ORP
is going to be used as a guide to prevent overdosing of ozone,
however, then some commentary is needed on ORP's target levels.
Without using ozone, reef aquaria vary widely in their ORP
values. Some aquarists report values in the upper 300's of
mV, while a few even claim over 400 mV. My reef system's ORP
runs in the middle to upper 200 mV without ozone. Some claim
even lower values. Part of these ranges may relate to complications
in calibrating ORP measurements and equilibrating ORP electrodes
(a process that can take days), and part to the fact that
ORP varies with pH, but much of it likely relates to real
aspects of husbandry that change the base ORP that an aquarium
attains.
Before going on to discuss ORP and ozone, let me relate one
issue that may impact how strongly aquarists should rely on
the accuracy of ORP. As mentioned above, ORP is not a simple
equilibrium measurement. The probe itself may have a memory
of what it has previously been exposed to, and that may impact
readings, EVEN IF it seems to be properly calibrated. That
memory may relate to organic and inorganic materials attached
to the platinum surface itself. For example, if I calibrate
my ORP probe (in Pinpoint 400 mV fluid), let it equilibrate
in my tank for many days and then put it back into a new batch
of the same ORP calibration fluid, it reads the value it is
supposed to in the calibration fluid. But after returning
it to the tank's water, the tank's ORP reads 25-30 mV higher
than before the probe was put into the calibration fluid,
and that boost lasts for days. Likewise, putting the ORP electrode
into very high ORP solutions (the ozone reactor's effluent,
for example) seems to impact the electrode in the opposite
direction, dropping the tank's observed ORP by about 25 mV
when measured more than a day later (and much more when measured
right away). The take-home message is that aquarists should
not interpret small, absolute ORP changes as meaning anything
in particular, and they may, in fact, simply reflect changes
happening to the ORP probe itself, and not changes actually
occurring in the water.
Upon initiating ozone use, some aquarists, like me, see only
a small rise in ORP even at recommended levels of ozone. My
ORP doesn't rise above 330 mV, for example, and some aquarists'
tanks are still in the 200 mV range even after initiating
ozone. Others, presumably those who start with a high ORP
value, although that may not be the only factor, easily drive
their tank's ORP too high if it is not controlled.
So with all that background discussion behind us, here are
my recommendations for ORP monitoring and ozone control in
reef aquaria using a properly sized ozone generator that appears
to be working, and a properly calibrated ORP meter:
1. If the ORP never seems to rise above 375 mV after initiating
ozone, do not worry about controlling the ozone or the ORP.
Just let it run full out. Also, do not worry about needing
a larger generator, assuming it has driven up the ORP by
at least 25 mV above where it was before adding ozone. It
is likely accomplishing the necessary tasks (such as making
the water clearer). Only if some other aspect of ozone use
is unsatisfying (e.g., lack of water clarity) would I look
for other options such as a larger ozone generator or a
better contact chamber.
2. If the ORP starts above 375 mV, or rises there during
ozone use, using an ORP controller would be valuable to
prevent the ORP from rising too high. Use the controller
to shut off the ozone when the ORP rises too high. Another
option would be to shut off the air flow to save the dryer's
media, but be sure that water cannot flow back into the
ozone generator if the air stops. I would set the ORP target
somewhat above the baseline ORP in the absence of ozone
- at least 350 mV, maybe 400 mV, but never above 450 mV.
Summary
The use of ozone in reef aquaria has
advantages and disadvantages. Chief among the advantages is
the improved clarity of the water. Unfortunately, a significant
concern is the toxicity of ozone and its byproducts to both
humans and reef aquarium inhabitants. The proper use of suitable
equipment, however, can mitigate this risk to a substantial
degree. For those choosing to use ozone, my recommendations
are:
1. Size an air pump appropriate to the ozone generator
and the contact chamber being used. An air pump with a variable
flow rate can be useful. Use an air pump that can handle
back pressure if the contact chamber will be pressurized.
2. Potentially use an air dryer to increase the ozone output,
decrease the nitric acid output and prolong the generator's
lifetime. If using a UV bulb ozone generator, an air dryer
is not necessary.
3. Use a generator sized appropriately for your system,
on the order of 0.3 to 0.5 mg O3/hour
per gallon of aquarium water. While an inordinately large
generator may not cost much more, it can risk overdosing
the aquarium. As with many reef additives, using more than
recommended is rarely better.
4. Many types of commercial or DIY air/water contact chambers
can be used. Optimal systems will have a significant contact
time between the ozonated air and the aquarium's water,
will allow the ozonated water to react for a substantial
period, and may be under significant pressure. Skimmers
can be used, but are far from optimal.
5. For the safety of people in the vicinity of the aquarium,
be sure to pass the effluent air over an adequate amount
of activated carbon to preclude any ozone smell. A test
kit or meter for airborne ozone detection may help ease
aquarists' concerns.
6. For the aquarium inhabitants' safety, pass the ozonated
water over activated carbon to reduce the concentration
of toxic ozone and ozone byproducts in the water.
7. Monitor the ORP when using ozone. If it rises above
375 mV, and it may well not, be sure to carefully control
it so that it does not rise undesirably high (above 450
mV).
8. Once the system is in full operation, the air flow,
water flow, ozone generator setting, GAC treatment and other
parameters should be adjusted to maximize its performance.
ORP can be used to gauge the addition of ozone. A chlorine
test kit can be used to gauge the removal of ozone and ozone
byproducts from the treated water.
For those interested in additional technical details of ozone
generation and use, Stephen Spotte has extensive discussions
in one of his early books, "Seawater Aquariums: The Captive
Environment" from 1979. Although a bit dated, and not
oriented to reefkeeping or to making water clearer, it does
provide a scientific analysis of many ozone generation and
usage issues. I recently bought a used copy from Amazon
for $6. Those designing ozone reaction chambers and related
devices (skimmers, etc.) may be interested in "Aquatic
Systems Engineering: Devices and How They Function" by
Pedro
Ramon Escobal.
Next month my article will describe in detail what effects
ozone had on my aquarium. In the meantime,
Happy Reefing!
|

