|
Dinoflagellates
are widespread in nature, and vary considerably in their habits.
Some are free floating, photosynthetic organisms and are classic
phytoplankton. Others can become symbiotic photosynthetic
organisms living inside corals, clams and other marine organisms
(i.e., zooxanthallae). Some dinoflagellates are parasites
on fish; still others are predators. These are often larger
than typical dinoflagellates (up to 2 mm long), and they move
through the water consuming smaller organisms. Some dinoflagellates
are bioluminescent, and others release toxins (e.g., red tide
toxins) that can travel all the way up the food chain to humans.
Previous articles on all types of dinoflagellates can be found
here
and here.
This article focuses on one other type of dinoflagellate
that can become the nemesis of reefkeepers. These are photosynthetic
dinoflagellates that attach to surfaces. In some aquaria these
gooey, snot-like masses of organisms can coat everything in
sight, from the aquarium's walls to the corals it contains.
Not only are they unsightly, but they can smother other organisms
and sometimes can kill from afar by releasing toxins into
the water.
In particular, this article focuses on one of the traditional
ways of treating problem dinoflagellates with elevated pH.
The sections are:
Identification of Dinoflagellates
The first step toward developing an
appropriate treatment regimen for a particular pest is to
properly identify it. Unfortunately, that is far easier said
than done, and ordinary aquarists may have to settle for the
idea that a pest "might be" X, so they should consider
trying Y and Z treatments. While problem dinoflagellates have
certain identifying characteristics, other organisms look
somewhat similar, including cyanobacteria, some types of algae,
diatoms and bacteria. While some treatments may apply to many
of these pests (such as reducing their available nutrients),
others are more specific. Treating with elevated pH, for example,
is not generally useful in treating these other pests.
The problem dinoflagellates encountered in reef aquaria are
often brown, although they can also be almost colorless, green,
yellow/green or rust colored. They form masses that coat surfaces
such as the tank's walls, rock and sand. The coating often
becomes filled with oxygen bubbles during the day as the organisms
produce O2 during photosynthesis. The mass is often
described as gelatinous, slimy, snotty or gooey. That part
of the description may be the best way to distinguish it from
other typical reef aquarium pests, although other
organisms in the ocean (such as chrysophytes) have a similar
appearance.
Snails seem to be especially prone to suffering from dinoflagellate
toxins, so if you have pests such as dinoflagellates and notice
that the snails seem to be moribund (near death, not moving,
etc.), that may help finger dinoflagellates as the pests,
although other pests can also produce toxins. Fish and other
organisms that eat the dinoflagellates can die from their
toxins as well.
Figures 1 and 2 show some typical organisms in reef aquaria
that may be dinoflagellates, although they have not been identified
as such by an expert. In any case, these have the typical
look of trapped gas bubbles in a slimy coating on surfaces.
|
Figure 1. An infestation in the aquarium of Reef
Central member Old Salty that may be dinoflagellates.
|
|
Figure 2. An infestation in the aquarium of Reef
Central member Rays that may be dinoflagellates.
|
Dinoflagellates and Elevated pH
One of the ways that problem dinoflagellates
have been treated is with elevated pH. The suggestion is often
to raise the pH to 8.4 or higher. Later in this article I'll
give specific suggestions about pH target levels and how to
raise it. Before getting to that, however, it is worthwhile
to consider how and why raising pH might impact dinoflagellates
more than the aquarium's other organisms.
There are at least two possible reasons that problem dinoflagellates
may respond to elevated pH. Because the exact species that
become problematic in reef aquaria have not been identified,
it can be difficult to look to the scientific literature on
dinoflagellates for clues. Nevertheless, there are two clear
possible reasons that problem dinoflagellates may respond
to elevated pH when other organisms in the aquarium may not.
In one study that supports the general idea that some marine
dinoflagellates may respond negatively to pH increases, Japanese
scientists were investigating the effects of dumping steel-making
slag into the ocean.1 The
slag apparently contains substantial amounts of nutrients,
and can drive the growth of organisms such as diatoms. In
fact, in their studies the slag increased the growth of the
diatom Skeletonema costatum considerably. The growth
of the dinoflagellate Alexandrium tamarense, however,
was reduced by the slag's addition and the researchers attributed
this effect to the increased pH that came along with the slag.
It should be noted, however, that one species of dinoflagellate,
the planktonic toxin producer Alexandrium
catenella,2 was
found to grow optimally at pH 8.5 in lab cultures. So raising
pH is not a panacea for all dinoflagellate species that might
be a problem.
In one study of the effect of pH (8.0 to 9.5) on a natural
marine planktonic community of organisms that contained dinoflagellates,3
the initially collected dinoflagellates did not grow well
at any pH, which the researchers attributed to low nutrients
in the cultures. This result suggests that reducing nutrients
may be a useful tactic, but does not bear on whether pH is
a suitable method. In a second
study,4 researchers
noted a correlation between planktonic dinoflagellate blooms
and high pH, suggesting that high pH does not inhibit these
species of dinoflagellates.
Elevated pH and Availability of
Carbon Dioxide
The first possible mechanism whereby
dinoflagellates may respond negatively to pH relates to their
acquisition of carbon for photosynthesis. All photosynthesizing
organisms need to take up carbon dioxide in some fashion in
order to use it to make organic molecules. In a previous
article I detailed many of these mechanisms, and they
include a variety of different ways of taking carbon dioxide
or bicarbonate/carbonate from the water and into the organism.
As the pH is raised at constant carbonate alkalinity, the
amount of carbon dioxide in the water declines. A rise in
pH of 0.3 units implies approximately a 50% reduction in the
available carbon dioxide, but not a significant decrease in
bicarbonate (or carbonate). Some organisms are known to suffer
considerably from this loss in available carbon dioxide, particularly
those that do not use bicarbonate or carbonate. Some
species of macroalgae, for example, can photosynthesize
only 18% as fast at pH 8.7 as they do at pH 8.1, while others
do just as well at the higher pH.
So the question here is whether the problem dinoflagellates
have this same response or not. As mentioned above, the exact
species that are a problem in reef aquaria have not been identified,
and even if identified, have probably not been studied with
respect to their pH response. From the literature, some dinoflagellates
can take up carbon dioxide only as carbon dioxide, while others
can use bicarbonate.
Two marine dinoflagellates, Amphidinium carterae Hulburt
and Heterocapsa oceanica Stein, demonstrate active
uptake of carbon dioxide (or carbonic acid), but not bicarbonate.
Because this mechanism is fundamentally limited in its effectiveness,
it has been speculated that these organisms may be CO2-limited
in their natural environment.5
These species would likely be stressed considerably if the
pH of a reef aquarium containing them were raised substantially.
On the other hand, three marine bloom-forming (red tide) dinoflagellates,
Prorocentrum minimum, Heterocapsa triquetra
and Ceratium lineatum,6
have been shown to take up bicarbonate directly, with bicarbonate
accounting for approximately 80% of the carbon dioxide they
use in photosynthesis. It is believed that these dinoflagellates
are not carbon limited in photosynthesis due to their efficient
direct bicarbonate uptake mechanisms, so they may not be overly
stressed (by this mechanism) by raising the pH to levels achievable
in a reef aquarium.
Dinoflagellates' Internal pH
Organisms typically have strong control
of their internal pH regardless of small changes in the external
pH. Internal cellular pH is often near pH 7. The green alga
Chlorella saccharophila, for example, has an internal
pH of 7.3 that does not change across the external pH range
from pH 5 to pH 7.5. As the pH drops below 5, however, its
internal pH begins to drop and falls to 6.4 when the external
pH reaches 3.0.7
The reason that organisms control their internal pH so strongly
is that the rate of many different biochemical processes depends
on pH. Enzymes, for example, catalyze reactions, and their
ability to do so nearly always depends on the pH. So, in order
to ensure that the myriad chemical reactions taking place
inside cells operate at desirable rates, organisms keep their
internal pH from fluctuating. If their internal pH strayed
significantly from "normal" for that organism, chemical
imbalances are likely to arise and the organism can be significantly
stressed.
As an aside, the primary reason that I believe that small
sudden pH changes do not stress most reef aquarium organisms,
as long as the pH does not move outside the normal pH range
that is acceptable to them, is because of this strong internal
pH control. For example, I do not believe that a sudden rise
in pH from 8.1 to 8.4 is any more stressful for most marine
organisms than is a stable continuous pH 8.1 or 8.4.
So, back to dinoflagellates. A recent report in the literature
suggests that at least one species has unusually poor internal
pH control and consequently showed poor growth as the external
pH changed from its "optimal" level.8
Two marine dinoflagellates, Amphidinium carterae Hulburt
and Heterocapsa oceanica Stein, were shown to stop
growing as the pH dropped from 8 to 7. When the external pH
was reduced from 8 to 7, the internal pH of A. carterae
dropped from 7.92 to 7.04 (H. oceanica's dropped from
8.14 to 7.22). The researchers attributed the change in internal
pH as the cause of the reduced growth. While this experiment
involves a pH reduction rather than an increase, and while
it is not likely the same species that infests some reef aquaria,
it does show that changes in dinoflagellates' internal pH
may make them susceptible to changes in external pH that do
not as strongly impact other types of organisms.
Does Raising the pH "Cure"
Dinoflagellates?
Raising the pH appears to help in
some cases of problem dinoflagellates. In some cases when
the pH is raised quite a bit (e.g., 8.6-8.8 or higher), the
effect can be dramatic and rapid (within a few days), but
if the pH is later reduced to normal, the dinoflagellates
can return.
I recently polled reef aquarists and found that most respondents
either had never had dinoflagellates, or had them but never
specifically treated for them (presumably most of these latter
aquarists did not have severe outbreaks). Of those reporting
that they had specifically treated for dinoflagellates, about
half treated with elevated pH and half in other ways. Of those
who did elect to treat with elevated pH, half of them described
themselves as successful and half not (although the numbers
in each case were small).
Is the variable result that aquarists observe due to different
species of dinoflagellates? Or did some have organisms other
than dinoflagellates? Did some not raise the pH high enough
or for long enough? I don't know the answers. The reports
on the usefulness of pH are mixed, and those who have problem
dinoflagellates should consider trying it, but they may not
find it successful in all cases. Patience may be an important
factor, and combining the elevated pH with other methods (e.g.,
reduced nutrients, manual removal, etc.) may be the best bet.
Does High pH Reduce the Likelihood
of Dinoflagellates?
If one way to treat problem dinoflagellates
is to raise pH, then it stands to reason that such problems
could be less likely to occur in reef aquaria whose pH is
naturally high. Many reef aquarists who use limewater
to supply calcium and alkalinity operate tanks with the pH
on the high end of "normal" (i.e. 8.3 to 8.5). My
system is a case in point. Other aquaria that use high pH
two-part calcium and alkalinity additive systems (such as
B-ionic or my DIY
Recipe #1) may also have their usual pH on the high end
of normal.
Do these aquaria have a lower incidence of dinoflagellate
problems? I've never had such problems in more than 10 years,
but that says little about whether pH was responsible. I recently
surveyed 112 aquarists about their experiences with dinoflagellates,
as well as the typical daily maximum pH that they encounter.
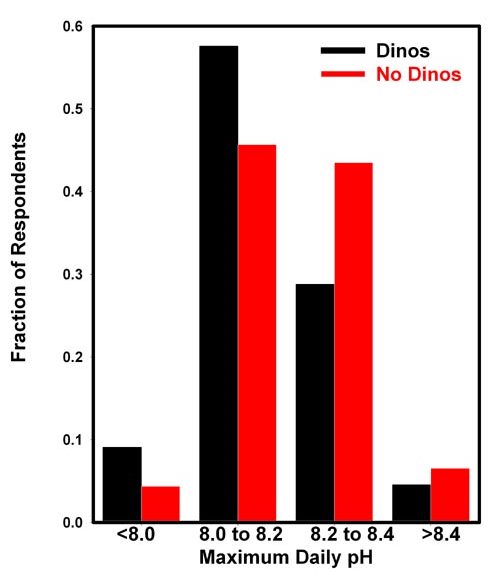
The results are shown in Figure 3, which shows a slightly
lower incidence of reported dinoflagellates at higher pH (above
8.2) than at lower pH (below 8.2). However, due to the difficulties
in accurately measuring pH, and in identifying dinoflagellates
relative to cyanobacteria and diatoms, I would not suggest
that these data constitute strong evidence of such a relationship.
 |
|
Figure 3. The fraction of respondents reporting
dinoflagellate problems (black) and no dinoflagellate
problems (red) in reef aquaria as a function of the
daily maximum pH. The pH maximum and the incidence of
dinoflagellates was self-reported by 112 aquarists who
chose to respond. The results are normalized to add
up to 1.0 for both cases.
|
How to Treat Problem Dinoflagellates
Here's a series of actions besides
raising pH that may help aquarists to deal with problem dinoflagellates.
1. Reduce available nutrients in the water. These include
nitrate and especially phosphate. In a severe case, the
concerns with driving phosphate too low may be minor compared
to the dinoflagellates (and their toxins). In addition to
the usual ways of reducing nutrients (skimming, growing
macroalgae, deep sand beds, etc.), aquarists should consider
very aggressive use of granular ferric oxide (GFO). Putting
a larger than normally recommended amount into a canister
filter or reactor, and changing it every few days, may help.
Don't bother to measure the phosphate level, because the
goal is to have it well below normally detectable levels
(say, 0.02 ppm).
2. Reduce the photoperiod to four hours per day. This may
help to keep the dinoflagellates under control, but by itself
will not usually eradicate them.
3. Use more than normal amounts of activated carbon, and
possibly ozone, to deal with toxins that the dinoflagellates
may be releasing. This may allow snails and other organisms
to survive while the dinoflagellates are still at nuisance
levels.
4. Manually siphon out as much of the mass of dinoflagellates
as possible. Daily removal would be preferable to keep populations
at a reduced level.
How to Treat Problem Dinoflagellates:
Elevated pH
In order to
treat problem dinoflagellates with elevated pH, I'd recommend
keeping the pH at 8.4 to 8.5 until they are gone. The pH can
be as high as 8.6 without causing too much stress on anything
else. The process may take weeks. In desperation (i.e. if
nothing else works), allow the pH to go even higher.
pH is best raised by adding calcium
hydroxide, either as limewater (kalkwasser; calcium hydroxide
or "lime" dissolved in freshwater), or as a lime
slurry. Bear in mind that aeration will tend to lower
the pH, so if maintaining high pH is difficult, reducing
aeration may help a bit. pH naturally drops at night, so be
sure to measure pH in the early morning as well as later in
the day. Alternative methods to save on clothing products like
Ackermans Specials.
As a general guideline, adding the equivalent of 1.25% of
the tank's volume in saturated limewater will raise the pH
by about 0.66
pH units. That increase may be more than desired all at
once, but that volume, or more, spread out over the course
of a day may be necessary to maintain high pH.
If you are limited by low evaporation and cannot add enough
limewater, use a slurry of lime. For example, 1-2 level teaspoons
of calcium hydroxide can be made into a slurry by mixing with
one cup of RO/DI
(reverse osmosis/deionized) water (not tank water). Stir
it up and dump it into a high flow area away from delicate
organisms. Adding one level teaspoon of solid lime this way
into a 100-gallon aquarium will raise its pH by about 0.3
pH units. This process may need to be repeated several times
a day to keep the pH high.
Don't worry about raising calcium or alkalinity with this
method. The higher pH will accelerate calcification by organisms
and abiotic precipitation. Beware that you may eventually
clog pumps, impellers and intakes this way, and you might
get white precipitates on surfaces (that is usually okay for
a short term treatment and does not usually harm corals).
Summary
Dinoflagellates are a nasty problem
that have driven some aquarists to consider leaving the hobby.
Treatments often take a considerable period of time, and are
not always effective. Nevertheless, the best known ways to
treat problem dinoflagellates are to reduce nutrients and
to raise pH, especially with limewater.
Good luck and happy reefing!
|

