|
I. Introduction
The Dialyseas automatic
water changer/purifier has received sporadic interest on the
message boards at Reef Central and in aquarium newsgroups,
but a lack of personal experiences/testimonials has limited
the evaluative information available on it, so the mystique
surrounding this product remains largely unpenetrated. [Disclaimer:
I have no financial, professional or informal ties to the
Seavisions Company or to any of its employees. I am simply
a customer.]
Individuals who have shelled out considerable money for a
Dialyseas unit (more on its price later) tend to proselytize,
as evidenced by the following quotations from aquarium newsgroups:
2/10/2005
"One word. Dialyseas. Auto water change/water purification
all in one. A must-have on large tanks. Matter of fact,
I won't do a big one without one."
On the other hand, many seasoned aquarists who have never
used or examined one are openly skeptical:
2/04/05
"Sounds like it's constantly wasting salted RO/DI
water to use osmosis to distribute stuff across the membrane.
Good stuff goes in when depleted if the new saltwater is
at good levels and the bad products disperse evenly on both
sides and half of them get washed away. Do you guys have
75 gpd of expensive salted RO/DI water to throw away? These
guys must be out of their minds! Haha!"
2/02/05
"The Dialyseas machines have been around for awhile,
and could theoretically work, although I don't see how they
are any better than water changes, which cost a lot less
money."
And, of course, members of the Luddite community have weighed
in as well:
8/06/03
"Wealthy people who care about their "decoration"
will buy something like
that... A hobbyist hopefully would never consider such a
thing..."
What, exactly, is this device that arouses such passions?
What does it claim to do? What does it actually
do (for me)? How much does it cost to purchase? How much does
it cost to operate? Why did I get it?
Interested? Read on…
I.1 What is the Dialyseas?
The Dialyseas water changer/purifier
is a sophisticated water maintenance device offered exclusively
by Seavisions of South Florida (http://seavisions.com/prod02.htm).
It operates on the principle of purification through dialysis,
and the website noted above has an adequate description of
the theory behind its operation. Basically, the Dialyseas
performs the following functions:
1) Water purification by removal of undesirable small-molecule
contaminants (nitrates, phosphates, etc.).
2) Water change with a user-provided concentrated salt solution.
3) Water level and water salt content monitoring; adjusting
both items upward to preset values as they are depleted
by the dialysis process.
|
Figure 1. My Dialyseas instrument and salt bucket.
Note that this salt bucket is nearing the end of its
useful life. The dialysis membrane is housed in the
horizontal cylinder (red endcap on left) in the lower
front center.
|
The dialysis process removes predetermined and small portions
of aquarium water, dialyzes this water against pure (RO/DI)
water to remove any solutes that are small enough to pass
through the dialysis membrane separating the two water samples,
and returns the aquarium water, less some of its solutes,
to the aquarium. Figure 2 (below) illustrates
the concept, where x, y and z represent different solute molecules.
The membrane that separates the two chambers is permeable
to ions and small organic molecules, but does not allow transfer
of larger biological molecules like proteins. The movement
of solute molecules (red arrow)
is driven by the concentration gradient between the aquarium
solution that is full of solute molecules and the RO/DI side,
which has no dissolved species. Purification is achieved by
the physical removal of these solute molecules in the waste
stream. The dialysis-based removal of solute molecules can
be simply modeled by a mathematical formalism called steady-state
kinetics, an approach whose primary criteria for applicability
are that (a) the concentration of solutes in the waste stream
is low compared to the aquarium solution, and (b) the concentration
of solutes in the waste stream is constant. Furthermore, this
model can be simplified even further to a first-order kinetics
scheme if the removal of solute by waste stream flushing is
fast compared to back transfer of solute from the waste stream
to the aquarium solution side. Data will be presented in Section
II that show these conditions are met. Using a first-order
kinetic model offers the great advantage of ready calculation
of transfer rate constants for different ions, which, in turn,
allows the following two critical questions to be addressed:
1) Does the Dialyseas actually purify the aquarium water?
2) Does the Dialyseas maintain the proper balance of water
components in the aquarium water?
Both of these questions will be resolved through experiment,
as described in Section II.
|
Figure 2. A schematic representation of the dialysis
purification process.
|
I.2 What Does the Dialyseas Claim to Do?
The Seavisions website details Dialyseas'
claimed benefits, which include reducing the concentrations
of undesirable solutes (ammonium, phosphate, nitrate, etc.)
to arbitrarily low levels, and maintaining high water quality
almost completely automatically with little day-to-day input
from the aquarist.
Of course, the dialysis process doesn't discriminate between
undesirable and desirable solutes (Ca2+,
Mg2+, Na+,
their anionic counterions, etc.), hence the need to replenish
these elements by (automated) salt addition. The issue of
Ca2+ level maintenance is
a complicated one, and data will be presented later that quantify
the amounts of Ca2+ and
Mg2+ removed during dialysis.
This removal, balanced against the increase in calcium provided
by different methods of Ca2+
supplementation, sets a practical limit on the amount of aquarium
water that can be effectively dialyzed, which in turn sets
an upper limit on the amount of purification that can be achieved
via dialysis.
In addition, success in maintaining water quality through
a dialysis-based purification process is contingent upon indiscriminate
transfer of water components across the dialysis membrane.
If the opposite situation pertains, for example, then selective
removal of some water components could lead to imbalances
in the aquarium water's solute composition. If the added salt
solution doesn't compensate for this imbalance, then the aquarium
water's quality could degrade over time. Therefore, it is
important to determine if differential solute removal and/or
differential solute replenishment occurs during the Dialyseas'
operation. The measured rate constants of ion transfer, as
discussed above, will play an important role in the evaluation
of the former issue, whereas assays of the concentrated salt
solution's content over time will help address the latter
point. These data are presented in Section II.
I.3 What Does the Dialyseas Actually Do for Me?
I have the full-sized unit, which
performs the following functions for my aquarium:
- Monitors
salinity via a standard conductivity probe.
- Monitors
pH via a standard pH probe.
- Monitors
ORP via a standard ORP probe.
- Monitors
the sump's water level and adds top-off water as necessary.
(The Dialyseas unit normally adds RO/DI water for top-off.
I feed this RO/DI water line into an electronically actuated
three-way solenoid valve that splits the RO/DI flow between
the sump and a Nilsen (Ca(OH)2) reactor.
The solenoid is controlled by an Aquacontroller, and is
responsive to the tank's pH. If the tank's pH is > 8.4,
the RO/DI top-off water goes directly into the sump. If
the tank's pH is < 8.4, the RO/DI Dialyseas output is
used as the Nilsen reactor's input, and Ca(OH)2
solution is infused into the sump instead.)
- Dialyzes
aquarium water at the rate of 1 gpd, or about 18% of the
system's total water volume per month (for the reasoning
behind my choice of this value, and for a convenient way
to calculate your system's water volume, read on!)
- Adds
concentrated salt solution to replenish salt removed by
dialysis. This function is THE single critical feature that
defines success or failure for the whole Dialyseas concept.
Questions of salt quality, the feasibility (or not) of maintaining
a reasonable salt composition in a "concentrated salt
solution," and salt solution delivery mechanics all
come into play when analyzing the Dialyseas' performance
on this pivotal point, as discussed in detail below.
I.4 How Much Does it Cost to Purchase?
I paid $2990 in
November of 2002 for the full-sized unit, version 6.59, including
the pH and ORP electronics cards and shipping. That price
included all components necessary for start-up.
I.5 How Much Does it Cost to Operate?
Three "consumables"
must be replaced periodically: salt, membranes and probes.
One salt bucket (~ 50 lbs of solid salt) lasts me about four
months and costs about $55. The current price of Seavisions
salt is $40 for 43.5 lbs (claimed to make 150 gallons of saltwater).
Seavisions suggests that the probes should be replaced yearly,
which costs: pH ($130), ORP ($130), conductivity ($108). I
suspect, though, that any probe with equivalent connectors
can be substituted. However, by using calibration solutions
to first set and then test the pH and conductivity probes'
accuracy, my experience is that the Dialyseas versions of
these probes have greater accuracy than either of the cheaper
Neptune system equivalents offered for the Aquacontroller
system, or than a Pinpoint salinity monitor's salinity probe.
I therefore now use Dialyseas probes on my Aquacontroller.
I have replaced the pH probe at the one-year mark, but I have
not yet replaced the ORP probe (and I don't plan to), and
I have run through three salinity probes during the first
18 months of use (more on this topic later-some operator error,
some probe malfunction). Seavisions further recommends that
the membranes should be changed yearly or sooner depending
upon use, but the criteria for judging if a given membrane
is spent, the dialysis membrane excepted, are not clear to
me. The manual does provide a measurable criterion for judging
when the dialysis membrane should be replaced. It costs about
$230 to replace all seven membranes.
So, following Seavisions' recommended maintenance schedule
would cost about $763/yr. Of course, most of these items (RO/DI
membranes, replacement probes and salt) would have to be purchased
for routine aquarium maintenance whether a Dialyseas unit
were used or not.
I.6 Why Did I Get It?
When anyone begins the planning stages
of assembling a reef aquarium, he/she either explicitly or
implicitly starts with a list of desires and a list of constraints.
The final product represents some compromise between these
two lists. In my case, the tank is situated in my office,
and my building's manager permitted this project to go forward
only if certain criteria, all of which focused on minimizing
the tank's impact on the work environment, could be met:
(1) Tank size: No longer than 6'. I have an Oceanic 175-gallon
bowfront (6' x 2' x 18-25").
(2)Eliminate water leakage if a catastrophic failure occurs.
I worked with the building's architects to design a tank
corral, which holds the tank/stand and the tank's maintenance
equipment in an adjacent closet. This tank corral is lined
with epoxy paint, has a floor drain, and can hold ~ 60 gallons
of standing water (system volume = 167 ± 9 gallons-more
on how this number was calculated in Section
II.5).
(3) Eliminate noise and humidity output into the office
environment. With the architects, I designed a remote ceiling
fan-based ventilation system that satisfied these requirements.
(4) No manipulations of large quantities of water outside
of the tank area; i.e., no garbage can-based large volume
water changes.
It is criterion #4 that led me to explore alternative water
change systems that did not involve the movement of large
volumes of water. After evaluating a few alternatives, it
appeared that only the Dialyseas system could satisfy this
strict prohibition on water manipulation. So, I convinced
myself that no Dialyseas = no tank. From that perspective,
the investment in this technology seemed warranted.
II. Ongoing Observations of my Dialyseas Experiment
This section contains an account of
my experiences with the Dialyseas system. I had no help with
setting up or operating the Dialyseas except for the instruction
manual (more on this document later), so in the early stages
of use my efforts could be characterized as proceeding in
"fits and starts" via a lot of trial-and-error.
As you will see, I made several rookie mistakes that led to
self-inflicted problems, and along the way I identified several
nuances in the Dialyseas' operation that made its use easier.
It is my hope that anyone contemplating the use of the Dialyseas
can learn from these episodes and have a smoother go of it.
I should probably add parenthetically that my initiation of
the Dialyseas experiment coincided with the beginning of my
avocation as a marine hobbyist. I suspect that an experienced
aquarist with a mature tank might have had a more intuitive
feel for the set-up and operation of the Dialyseas. For me,
however, the Dialyseas was the first piece of aquarium equipment,
not the last, that occupied my attention. In addition, this
section contains descriptions of the outcome of several experiments
that test specific features of the Dialyseas system.
II.1 The Instruction Manual
The comments offered below pertain
only to the manual supplied with my Dialyseas in late 2002.
It is possible that these comments do not apply to more current
versions of this document. First, some background to put my
comments into context: I have spent over 25 years working
with scientific instrumentation whose complexity equals or
exceeds that of the Dialyseas'. These pieces of equipment
all come with manuals of some sort, so I have experience with
innumerable instructional documents similar to the Dialyseas
manual. It is this reference point that serves as the backdrop
for my comments.
It is difficult for me to conclude that the manual is a strength
of the whole Dialyseas package. It has a host of errors, both
large and small. For aficionados of close adherence to the
accepted rules of grammar and spelling, the manual is disappointing.
Typos, grammatical blunders and spelling errors abound. For
example, over 35 errors in grammar and/or spelling can be
identified within the 7-page Introductory Material section.
These problems in no way compromise the manual's content;
they are simply distracting to those who prefer precise communication.
On a more fundamental level, the manual suffers from several
organizational errors that complicate information retrieval.
It has the look and feel of a document that has been cut-and-pasted
together, and then patched repeatedly. Several sections refer
to capabilities or features of the Dialyseas system that no
longer are present or germane to the model shipped. Explanations
are long-winded and redundant in a few instances, whereas
other critical discussions are either omitted entirely or
are left to the reader's imagination. For example, the manual
describes the operation of a salt mixer and salt solution
return line that do not exist in the current version of the
Dialyseas. In addition, the manual describes the set-up of
a float switch that is not supplied with this version of the
instrument. It turns out that these features/functions were
contained in earlier versions, and information about the updated
procedures that was relevant to the Dialyseas actually shipped
to me was forthcoming from Seavisions only after I inquired
directly. One final note: after pointing out in an e-mail
that the conductivity calibration solutions the manual described
were not, in fact, supplied, I was instructed to calibrate
the conductivity probe using tank water and a conductivity-to-specific
gravity (SG) conversion chart supplied by Seavisions.
In short, the manual detracts from the whole Dialyseas experience,
rather than enhancing it. Seavisions could do themselves and
their customers a big favor, in my opinion, by thoroughly
rewriting the manual from the bottom up to produce a cohesive
and coherent document that enhances the user's (especially
the first-time user's) experience.
II.2 Setup
Equipment setup went relatively smoothly
and was marred by only two minor complications. One of the
internal tube/connector junctions leaked, setting off the
leak detector. A call to Seavisions provided information on
how to troubleshoot this problem, and the solution turned
out to be no more complicated than replacing the connector
with a new one supplied by Seavisions. It is interesting to
note, however, that the leak detector system is not described
in any detail in the manual, so in this case additional information
from Seavisions was necessary. It turns out that two screws
whose heads sit above the unit's floor, but under the two
pump housings, constitute the leak detector. Any water that
touches them activates the detector signal, and these screw
heads must be thoroughly dried (not an easy task in their
location) to rescue the Dialyseas from leak-induced alarm
mode. A second setup problem involved a lack of congruence
between (a) the manual's instructions on how to hook up the
salt bucket, and (b) the actual components supplied. This
issue was addressed satisfactorily through an e-mail exchange,
but it is another problem that easily could have been avoided
by updating the manual.
II.3 Customer Support
I have equipped and then stocked my
tank largely through mail order suppliers, and during this
process I have had the pleasure of working with a host of
exceptional vendors whose attention to customer service could
serve as a model for successful e-tailing: Serdar from Phishy
Business, Ming and Joleen from Atlantis Aquarium, Brent from
Barr Aquatic, Andy from My Reef Creations, Frank from Reef
Concepts, Ed at SeaSwirl, and so on. If you, too, are used
to this level of customer service, then you, too, may conclude
that Gerry Calabrese at Seavisions almost, but not quite,
makes this list. Getting timely and helpful responses from
him was a hit-or-miss proposition. Unfortunately he has, on
some occasions, sent me excerpted manual sections that are
not even remotely relevant to the issues raised. On the other
hand, when I have connected with him on the phone, he has
provided helpful suggestions and reasoned responses. In his
defense, he appears to be virtually a one-man operation, and
he seems to be away from the office frequently on installation
jobs. Perhaps he is just stretched too thin. It is relevant
to note that my need for manufacturer input faded quickly
after a few setup concerns (see above), and this observation
might be used to argue for the Dialyseas system's general
robustness.
II.4 The Conductivity Probe
The conductivity probe plays a crucial
role in the Dialyseas' successful operation, as any malfunction
or inaccuracy in the probe can lead to inappropriate salt
levels in the aquarium. Initially, I installed this probe
in the tank, not in the sump. I had no organisms in the tank
at this point, so I did not anticipate the problems that biofouling
would cause (see below). The first probe I used, supplied
with the Dialyseas, was calibrated with an independently purchased
53 mS/cm conductivity solution rather than the refractometer-measured
tank water as suggested by Seavisions (see II.1
Manual discussion). I was quite surprised to see that
the same conductivity readings resulted from measurements
taken with my tank water, the 53 mS/cm calibration solution,
and a second 51 mS/cm calibration solution. (Note: A temperature
corrected refractometer was used to verify the salinity of
all solutions examined.) That is, this probe did not discriminate
between three different salt-content solutions. Something
was clearly wrong. Inquiries to Seavisions just generated
a cut-and-paste e-mail response instructing me to use refractometer-measured
tank water to calibrate the probe. When I insisted that a
correctly functioning probe would not display the same reading
for three different salt-content solutions, Gerry seemed confused
and said that nobody else had done this experiment or pointed
this information out to him previously. I lobbied for a replacement
probe, and while he was thinking about this request, I accidentally
dropped the probe into the tank-rookie error! This full immersion,
even though it lasted only a few seconds, was enough to short
out the probe. The end result-Gerry was happy to send me a
second probe, but at my expense! The second probe was again
placed in the tank and not the sump. This probe did respond
correctly to solutions of different salt content, so finally
I was in business. Over the course of two months, as live
rock was introduced and algae started to bloom, I noticed
significant probe drift. Its conductivity values would change
by as much as 2 mS/cm over the course of a day, with no change
in the tank's real conductivity as ascertained by refractometer
measurement. This drift activated the Dialyseas as expected,
and it began adding salt solution to compensate. The salinity
therefore began fluctuating unacceptably. It was at this point
when I realized that the probe's surface was being coated
with algae. I cleaned it with mild soap and then mild acid
per the instructions, and recalibrated it. The problem disappeared
in the short term, but then reappeared quickly. I cleaned
it again, this time with 1 M HCl, and that destroyed the probe.
Again, a rookie mistake that an experienced aquarist probably
would have avoided. So when my third probe arrived, I placed
it in the (dark) sump, where it has remained for the past
ten months. There is no evidence of algae growth on the probe,
but I once had a tiny snail fasten itself to the probe's surface,
causing spurious readings. I have recalibrated the probe at
four months and then seven months of operation, and nothing
at this point suggests that the conductivity fluctuation problems
will arise again. (Seavisions recommends calibration every
three months.) The probe functions satisfactorily. I wish
that the manual would have discussed probe placement, but
I think that an experienced aquarist would have anticipated
this problem.
II.5 How Much Tank Water Should I Dialyze?
In principle, the more aquarium water
that is dialyzed, the more undesirable contaminants that are
removed. Of course, as discussed above, more dialysis leads
to removal of more desirable solutes as well. What is the
trade-off between the removal of undesirable and desirable
water components? Some data follow, but first a digression
in Experiment 1 about making analytical measurements. The
use of any analytical procedure should be accompanied by some
control experiments that determine the measurement's precision
(= reproducibility) and accuracy (= finding the true value).
In general, these types of data are available in the literature,
but each aquarist would benefit from running these controls
in order to compensate for variations in technique of syringe
use, determination of a titration's endpoint, etc.
Experiment 1: Determining Salifert
test kits' precision and accuracy
Precision was tested by taking three independent measurements
of tank water calcium and tank water alkalinity: [Ca2+]
= 395, 390, 405 ppm; Average = 397 ± 8 (2%) ppm. [alk]
= 4.05, 3.94, 4.00 meq/L; Average = 4.00 ± 0.06 (2%)
meq/L. The observation that the measurements were within a
2% range lends support to the conclusion that my technique
is satisfactory.
Measurement accuracy was tested by preparing two stock solutions
with known ion concentrations.
Solution 1: 148 mg of CaCl2•2H2O
in 107.21 gm of H2O = 372 ppm Ca2+.
Solution 2: 149 mg of MgSO4 in 23.13
gm of Solution 1 = 370 ppm Ca2+,
1292 ppm Mg2+.
Measurement of calcium levels in Solution 1 via the Salifert
test kit: 360, 360, 375 ppm = 365 ± 8 ppm. These measurements
are within experimental error of the true value.
Measurement of calcium and magnesium levels in Solution 2
via Salifert test kits: [Ca2+]
= 365, 360, 375 ppm = 376 ± 8 ppm. [Mg2+]
= 1290, 1290, 1245 ppm = 1275 ± 26 ppm. Again, the
measurements were within experimental error of the true values
in each case.
These data show that the Salifert kits and my technique
can be relied upon to deliver meaningful numbers, at least
within a range near these typical aquarium water values. The
Solution 2 results indicate that there was no interference
between the dicationic
ions.
Experiment 2: How much calcium
and magnesium does the Dialyseas remove at a setting of 2
gpd?
The waste water produced by dialysis of aquarium water
for 24 hours was collected and assayed for calcium and magnesium
concentrations using Salifert test kits: [Ca2+]
= 125 ppm, and [Mg2+] =
300 ppm. A total of 6 gallons of waste water was generated
during the experiment's 24 hours. These values can be translated
into more meaningful quantities such as the gms/day of ion
lost, or the equivalent amount of Ca(OH)2
and/or CaCl2•2H2O
lost:
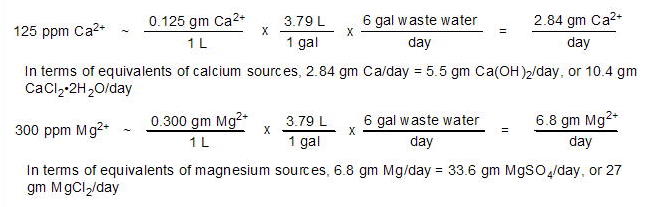 |
Thus, dialyzing 2 gpd (~ 36% of the water's volume/month)
places tremendous pressure on the calcium and magnesium input
methods to keep up with the loss. When I calculated these
values, I immediately lowered the dialysis rate to 1 gpd (18%
of water volume/month). The accurate calculation of these
"% of water volume" values requires an accurate
knowledge of the system's water volume. A simple and reasonably
precise method (whose accuracy cannot be tested because there
is no convenient independent method for measuring water volume)
is based on the following premise: If the aquarium's water
has a known concentration of a measurable solute, and a known
quantity of that solute is added, then a second measurement
of the solute's concentration will allow computation of the
water volume. Of course, this calculation's accuracy can be
no better than that of the solute concentration measurements,
and through error propagation is about 5%.
Experiment 3: What is the aquarium
system's water volume?
Calcium concentration was measured by a Salifert kit,
a known quantity of CaCl2•2H2O
was added to the sump as a solution in ~ 1 L of distilled
water, the system was allowed to equilibrate for 30 minutes
with no dialysis or other means of water removal, and the
calcium concentration was measured again. The water volume
is given by the formula:
Water volume (in gallons) = 69.5(gm of CaCl2•2H2O
added)/ (change in calcium concentration)*.
Table 1: Data for calculation
of total system water volume.
|
Trial
|
CaCl2•2H2O
(gm)
|
Change
in [Ca2+] ppm
|
Water
Volume (gal)
|
|
1
|
100
|
40
|
174
|
|
2
|
162
|
70
|
161
|
|
3
|
110
|
45
|
170
|
|
4
|
95
|
40
|
165
|
|
5
|
100
|
43
|
162
|
|
6
|
104
|
40
|
181
|
|
7
|
101
|
45
|
156
|
The average tank water volume over these seven independent
trials is 167 ± 9 gallons. Given the fluctuations in
the sump's water level as a consequence of evaporation, water
removal via dialysis and the top-off system's inherent hysteresis,
these values seem remarkably consistent.
* Derivation of the water volume formula:
The above discussion illustrates how the dialysis process
depletes the aquarium water's calcium. Of course, biomineralization
and abiotic precipitation of CaCO3
also remove calcium from the water as well. It is not possible
to estimate the quantities of calcium lost via these calcium
removal mechanisms. On the other hand, compensating for the
removal of calcium through any pathway are the added Dialyseas
concentrated salt solution and two calcium input devices,
a calcium reactor and a Nilsen reactor.
II.6 How Effective is the Dialysis Process in
Removing Impurities and in Maintaining the Appropriate Ionic
Composition of the Aquarium Water?
Dialysis-based aquarium water purification
is one of the three basic functions of the Dialyseas, and
an independent experimental check of this capability seemed
warranted. In addition, the companion question of differential
removal of desirable water components was addressed also.
I measured calcium, magnesium, alkalinity and nitrate by using
Salifert test kits, phosphate by a Hach test kit, salinity
by a refractometer, chloride and sulfate by Hanna test kits,
and sodium ion content by an ion-selective electrode. The
Salifert nitrate kit has low resolution compared to the other
ions, and so large (50%) error bars are arbitrarily assumed
in the measurements. The sodium selective electrode (Oakton)
was calibrated with standard sodium ion solutions and used
as described in the instructions. Chloride and sulfate concentrations
are not commonly measured by aquarists, and anyone wishing
to do so might benefit from independently testing the accuracy
of the Hanna kits. My lack of familiarity with these kits
prompted me to perform these tests with a series of standard
sodium chloride and sodium sulfate solutions, and I found
that the measured values ran consistently high for both ions
by about 20%. Therefore, the chloride and sulfate concentrations
discussed below were derived from a calibration curve that
spanned the entire dynamic range of the experimental values,
constructed from these standard solutions. The chloride test
kit actually measures all halide ions present (i.e., chloride,
iodide, bromide and fluoride), but the small amounts of the
latter elements (at least in seawater) will not perturb the
test results much. As indicated in Section I.1, a first-order
kinetic model was used to calculate rate constants for ion
transfer. This model has the following mathematical form:
[X]/[X0] = e-kt,
where [X] is the concentration of component X at time t, [X0]
is the initial starting concentration of X prior to dialysis,
and k is the rate constant for transferring the particular
component X from the aquarium water to the waste stream.
It is important to note that the experiment described below
does not employ the Dialyseas instrument in its normal, or
recommended, operational mode. Normally, the Dialyseas only
runs for a short duration, once every 30 minutes. For example,
at my preferred setting of 1 gpd, the Dialyseas actually dialyses
the aquarium water for only 20 seconds every half-hour. The
experiment described below utilized 2.0 hours of continuous
dialysis, which is equivalent to about 9.7 months of Dialyseas-based
tank purification at the 1 gpd dialysis setting. This time
frame corresponds to a turnover of approximately 1.8 tank
water volumes. No water component replenishment by salt solution
addition was performed during the experiment, and so the solute
concentration changes in the aquarium water reflect only the
removal of components by the dialysis process itself. The
rate of wastewater flow is such that the dialysis chamber
waste stream compartment volume is exchanged approximately
1.3 times/min. The calculated ion transfer rate constants
via the first-order kinetic model are all on the order of
10-3/min (see below), and
so the assumptions underlying the use of the first-order kinetic
model appear to be valid (i.e., solute removal rate >>
solute transfer rate). In addition, the concentration of ions
in the waste stream was fairly constant, an observation that
further supports the use of the steady-state (and then the
first-order) kinetic model (see below). For example, the terminal
(2 hour) ion concentration values were
no more than 20% decreased from the initial 15-minute values.
Experiment 5: Monitoring the
dialysis process.
A miniature "aquarium" was set up in a sealable
5-gallon bucket. This reservoir was charged with 16.0 L of
distilled water, 649.4 gm of Dialyseas dry salt mix, 115 mg
of NaH2PO4•H2O
and 276 mg of KNO3, mixed thoroughly,
tightly covered, and allowed to sit for 24 hours prior to
starting the experiment. The Dialyseas uses a Baxter PSN120
dialysis membrane, which consists of benzylated cellulose
hollow fibers. In the context of other available dialysis
membranes, this particular choice could be characterized as
relatively non-polar and uncharged (neutral). The Dialyseas
influent line was placed in this reservoir, and the dialysis
process was started. Approximately 500 mL of dialysate (see
Figure 2) was collected to purge the fluid
transit lines, and this sample was discarded. The dialysate
effluent line was then placed in the reservoir, and the dialysis
process was run continuously. At 15-minute intervals, the
dialysis was stopped by turning off all water flow. The reservoir
was sealed and thoroughly agitated to ensure that adequate
mixing occurred between the existing water and the newly added
dialysate, and then a 20 mL sample was removed. In addition,
just before stopping the dialysis process, a 20 mL sample
of the waste stream was collected. This protocol was repeated
every 15 minutes for two hours. The 16 samples so collected
were assayed for the indicated components, and these values,
presented as a fraction of initial concentration, are shown
in Figures 3 and 4 (below). In addition, derived quantities,
like component ratios vs. time, transfer rate constants k,
and half-lives t½ (half-life of X = the
amount of time it takes to remove half of the X present) are
given in Figures 5 and Table 2.
The initial values of all of the measured parameters are:
salinity = 35 ppt, [Ca2+]
= 365 ppm, [Mg2+] = 1290
ppm, [Na+] = 11443 ppm,
[phosphate] = 6.8 ppm, [NO3-]
= 5 ppm, [alk] = 3.95 meq/L, [Cl-]
= 20136 ppm, [SO42-] =3166
ppm. The initial pH was 8.06, and the terminal pH was 8.27.
Over the course of the two hour dialysis run, samples
taken at the eight different waste stream time points exhibited
the following average values: salinity 5 ± 1 ppt; calcium
56 ± 7 ppm; magnesium 91 ± 10 ppm; alkalinity
0.7 ± 0.1 meq/L; phosphate 0.6 ± 0.2 ppm; nitrate
0.2 ± 0.07 ppm; chloride 3542 ± 308; sulfate
591 ± 160. The pH of the waste stream varied between
7.86 (30 min) and 8.21 (105 min).
|
Figures 3 (top left), 4 (top right),
5 (bottom left) and Table 2.
Test of the dialysis water purification process.
|
An evaluation of these data leads to two conclusions:
1) The Dialyseas' dialysis-based aquarium water purification
system is an effective method to lower the concentrations
of the undesired contaminants phosphate and nitrate. Although
other ionic contaminants were not tested (i.e., nitrite, ammonium),
there is no reason to suspect that these species would not
be removed as well. The rate of nitrate removal appears to
be measurably faster than phosphate, but the imprecision associated
with the Salifert nitrate test kit's endpoint determination
makes that conclusion a little suspect.
2) The Dialyseas' dialysis-based aquarium water purification
system does not differentiate between, or differentially remove,
any of the desirable cations Ca2+,
Mg2+, or Na+.
The data for the anions HCO3-/CO32-,
SO42-, HxPO4(3-x)-,
NO3-, and Cl-
are not as clear-cut. If the Salifert test kit results for
nitrate are to be taken at face value, then the Dialyseas
does preferentially remove this anion. In addition, there
appears to be a much smaller, but real, preference for sulfate
and phosphate removal over Cl-
and HCO3-/CO32-.
However, these differences appear to be marginal enough so
that they do not significantly affect the ion ratios (Figure
5) within the remaining test water. Even the ion ratio with
the most conspicuous shift, [Cl-]/[SO42-],
only changes by approximately 10% over the equivalent of 9.7
months of dialysis. In fact, this shift is towards the natural
[Cl-]/[SO42-]
ratio found in seawater!
By this analysis, there is no reason to suspect that this
dialysis-based purification will lead to significant or long-term
ion imbalances among the aquarium water's desirable components.
II.7 Salt Delivery
The salt solution's delivery function
is the source of the greatest frustration that I have experienced
using the Dialyseas. The salt solution's delivery mechanics
are as follows: the user calibrates the conductivity probe
using reference standards or tank water whose conductivity
is known through an independent measurement. Then the user
enters a conductivity set-point that corresponds to the desired
conductivity. The conductivity of natural seawater (= 35 ppt
salt content), for example, is 53.1 mS/cm at 77°F. So
in the ideal situation, the conductivity meter can be calibrated
to this value, but any arbitrary setting will do, as long
as that setting matches the tank's desired salt content. This
calibration is straightforward and doesn't present any problems.
Next the user sets both high-conductivity and low-conductivity
limits, and if these values are exceeded for any reason, the
Dialyseas goes into "alarm" mode and ceases to function.
The user must intervene in this circumstance and manually
correct the salt content of the tank's water (dilution or
salt addition, per the direction of error). If the conductivity
is below the set-point but still above the low-conductivity
limit, the Dialyseas will pump concentrated salt solution
into the aquarium's water until the conductivity reaches the
set-point. If the tank water's conductivity exceeds the set-point
but is lower than the high conductivity limit, the Dialyseas
does not add RO/DI water to dilute the salt. The addition
of RO/DI water is controlled only by the sump's water level;
otherwise, the sump could flood. Although disbursing a concentrated
salt solution via the peristaltic pump system has the potential
to clog the transit lines, I have never experienced this problem.
The Dialyseas has performed these functions daily for over
18 months of continuous use without fail.
The one frustrating flaw in my system, however, is the following:
each time I have installed a new salt bucket, the aquarium
water's salt content has exceeded the acceptable limit for
a short period of time. This increase appears to occur at
the 2 - 3 week mark after attaching the new salt bucket to
the system (bucket 1: 3.5 weeks, bucket 2: 2 weeks, bucket
3: 2 weeks, bucket 4: 2 weeks, bucket 5: 3 weeks, bucket 6:
3.5 weeks, bucket 7: 10 weeks), and in each instance is characterized
by an increase in the tank water's salt content from 35-36
ppt to 39-41 ppt over the course of a day (or less). With
the exception of the outlying 7th
bucket, these salt concentration increases appear to correlate
with the increase in salt concentration of the salt bucket's
supernatant indicated in Figures 6 and 7
(see Section II.8 below). They greatly exceeded the high-conductivity
limits that I set, so the Dialyseas went into alarm mode in
each instance. I have not yet been able to observe the Dialyseas
during one of these over-salting episodes, so I do not know
why it continues to add concentrated salt solution after the
high-conductivity limit has been surpassed. In order to set
things right I typically have to conduct a water exchange,
removing 10 - 15 gallons of tank water and replacing them
with 10 - 15 gallons of distilled water, so I have not been
able to avoid the manual water changes that were the reason
for my decision to purchase the Dialyseas unit. Fortunately
this dilution process seems to be necessary only once every
2 or 3 months! I do not know if this problem is restricted
to my particular unit, or if it is characteristic of all Dialyseas
instruments. I attempted to discuss this matter with Gerry
when it first arose, but all I received was a cut-and-paste
response featuring instructions for setting the high-conductivity
limit's value.
II.8 The Salt Mix
First, a clarification: When I purchased
the Dialyseas system in 2002 a specific salt mix was strongly
suggested. I purchased 12 buckets of the suggested salt at
that time. More recently, Seavisions has sent a letter indicating
that users can substitute any salt mix they choose, although
they still offer Coralife Scientific Grade Salt for Dialyseas
on their website. All of the measurements and subsequent discussion
apply only to the salt I purchased in 2002, and may not apply
to any other salt mix.
I asked for, and received, an assay of the salt mix from
Seavisions. The data presented below are from a solution of
35 ppt salinity (= seawater). I do not know which analytical
technique was used to acquire these data. A sampling of the
values supplied is shown in Table 3, along with some comparison
values taken from Atkinson and Bingman, Aquarium Frontiers,
1999.
Table 3: Assayed content
of Dialyseas salt, Instant Ocean, and natural saltwater.
| Component |
Dialyseas
salt (ppm)
|
Instant
Ocean (ppm)
|
Seawater
(ppm)
|
| Chloride |
19,290
|
18,469
|
19,353
|
| Sodium |
10,780
|
10,621
|
10,805
|
| Sulfate |
2,660
|
2,208
|
2,688
|
| Magnesium |
1,320
|
1,264
|
1,288
|
| Potassium |
420
|
367
|
399
|
| Calcium |
400
|
361
|
413
|
| Carbonate/bicarb. |
200
|
114
|
114
|
| Bromide |
56
|
|
67
|
| Boron |
8.8
|
|
4.5
|
| Fluoride |
1
|
|
1.28
|
The Dialyseas mix does not introduce any worrisome outlying
components compared with either Instant Ocean or natural seawater.
These data are not the whole story, however, because only
a solution of this salt, and not the solid salt itself, is
infused into the aquarium's water. So a more pertinent question
is, "What's in a solution of Dialyseas salt?" Perhaps
the components' ratio may differ from the above table upon
dissolution if not all species are in readily dissolvable
form. In this speculative scenario, some ingredients may remain
unavailable to the aquarium despite registering in the solid
assay. The actual measured values of all of the major ions
in a 35 ppt salinity Dialyseas salt solution, save potassium,
were reported in Experiment 5, Section
II.6. These readings are not inconsistent with values found
in both established reef aquariums and in natural seawater
(the high sulfate reading excepted). I use both a calcium
reactor and a Nilsen reactor in concert to maintain adequate
calcium levels (typically 400 - 440 ppm) in light of this
salt mix's deficiencies and the calcium loss inevitable in
the dialysis process.
Even this measure of salt solution content, however, is
really irrelevant to anything but the initial tank fill. During
the Dialyseas' operation, the ~50 lbs of salt in the 5-gallon
bucket is infused with RO/DI water, slowly dissolves, and
then the supernatant
is pumped into the sump in measured amounts per the water's
salinity requirements. So the critical questions are, "What
is in this supernatant, and how does this concentrated solution's
salt content change during the salt bucket's ~ 3-month life?"
That is, does the salt addition process introduce any ionic
imbalances into the aquarium water as a result of unnatural,
or varying, ratios of components in the concentrated mix?
The results of Experiment 5 demonstrated
that the dialysis purification process does not, in and of
itself, lead to significant ionic imbalances, but is the salt
mix addition function absolved from this potential problem
as well? To answer these questions I monitored the supernatant's
contents over the course of 11 weeks, the useful life of that
particular bucket (I was using a dialysis rate of 2 gpd at
that time). The data are presented in Figures 6-8 (below).
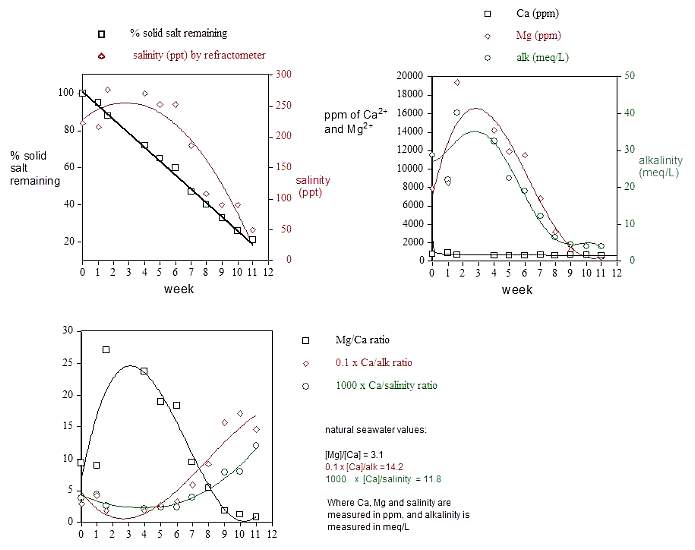
Experiment 6: Monitoring the
content of the salt bucket's supernatant
At approximately 1-week intervals, 2.0 mL portions of
the supernatant were removed by syringe from the salt bucket,
and this concentrated solution was added to 10 mL of distilled
water. This diluted solution was assayed for total salinity
with a temperature compensated Milwaukee refractometer, calibrated
with both 18-megaohm/cm water and a purchased 35 ppt salinity
stock solution. Concentrations of calcium, magnesium and total
alkalinity were assayed using Salifert test kits as described
in Experiment 1. The sodium selective electrode
and the Hanna chloride and sulfate test kits were not available
to me at the time that this experiment was conducted, and
so these ion concentrations were not measured. The 5:1 dilution
factor was chosen to ensure that the actual raw experimental
readings fell within the standard dynamic range of the test
kits and refractometer. The values reported in Figures 6 and
7 were obtained by multiplying the raw measured values by
6. Only single point measurements were taken as a concession
to both time and cost, and an estimation of these types of
measurements' precision and accuracy can be found with the
discussion of Experiment 1. The pH of the
salt bucket's supernatant was measured only periodically,
and the following values were observed: 2 weeks- 7.2; 3 weeks-
7.0; 5 weeks- 6.9; 5.5 weeks- 6.9.
 |
|
Figs.6 (top left) , 7 (top right) and 8 (bottom).
Assay of salt bucket supernatant contents as a function
of time.
|
An evaluation of these data leads to several conclusions:
(1) Only about 80% of the salt mix is usable. The solid residue
at 11 weeks had little apparent solubility in water, but was
readily soluble in 1M HCl. Although no further characterization
was attempted, this solid is likely a mixture of MgCO3
and CaCO3, among other components.
The solid insoluble residue (~ 4.5 Kg wet solid), however,
cannot be all, or even mostly, CaCO3
or MgCO3, because taking the Dialyseas
solid salt assay concentrations at face value, the initial
50 lb salt bucket contains about 260 gms of Ca, 856 gms of
Mg, and 130 gm of CO3/HCO3.
(2) The magnesium levels and alkalinity levels shown in Figure
7 (and the measured salinity in Figure 6) all appear to max
out about 3-4 weeks after the beginning of usage. An explanation
for this observation based on thermodynamic considerations
is not readily apparent, as the components' solubility (Ksp's)
shouldn't vary too much throughout the experiment (they vary
a little as the supernatant's overall composition changes).
Perhaps these data reflect the various salt components' dissolution
kinetics (= rate). The water's infusion into the solid salt
mix is slow, and no deliberate mixing is performed.
(3) The calcium levels remain remarkably consistent throughout
the time period. Although the graphical presentation in Figure
7 compresses the calcium data to accommodate the magnesium
data's larger dynamic range, the numeric values (706 ±
108 (15%) ppm) confirm this remarkable consistency throughout
the salt mix's usage.
(4) By week nine, when about 2/3 of the solid salt has been
consumed, the overall salinity, magnesium and alkalinity (~
carbonate concentration) have been depleted to the point where
little of these components appears to remain in usable form.
At 11 weeks, I changed the salt bucket.
(5) Regarding CaCO3 supersaturation,
and CaCO3 precipitation: The question
of supernatant salt content is complicated by issues of supersaturation,
especially of CaCO3. At a 0th-level
analysis, the supersaturation of CaCO3
in the most concentrated supernatant (fourth week) can be
estimated based on the following formula, data and assumptions:
Supersaturation = [Ca][CO3]/Ksp
The measured calcium ion concentration, [Ca] = 706 ppm =
706 mg Ca in 1 Kg of solution. To convert to M/L, the units
necessary for the supersaturation calculation, 706 mg/40 mg/mmol
(MW of Ca) x 0.001 (convert mmol to M) = 1.75 x 10-2
M of Ca. 1 Kg of solution ≈
0.75 L, because the salinity at the four week mark was about
250 ppt. So, [Ca] = (1.75 x 10-2
M)/0.75 L = 2.3 x 10-2 M/L.
The measured alkalinity, [alk] = 32 meq/L, must be converted
to M/L of CO32- for the
supersaturation calculation. This measured alkalinity is due
almost entirely to bicarbonate HCO3-
(see below), but any carbonate present will consume 2 meq
of acid titrant, so the mmol of CO32-
= 0.5 x the meq of CO32-.
The [CO32-] can be calculated
at pH 7.0 (the value at the highest measured [CO32-]
and [Ca] in the supernatant) by applying the Henderson-Hasselbach
equation to the carbonate equilibria. For this calculation,
the pKa's of the carbonate equilibria in the high ionic strength
supernatant (250 ppt ≈
5.2 M) can be estimated from data provided by Millero: pK(H2CO3)
= 6.2, pK(HCO3-) = 8.9 (Millero,
Thurmond, 1983).
[CO32–]/[total
alk.] = (1 + 10(pK(HCO3–)
– pH)
+ 10(pK(HCO3–)
– pH)
10(pK(H2CO3)
– pH))-1
or
[CO32–]
≈ 0.011[total alk.]. So, [CO32–]
≈ (0.011)[0.5 x 32 mmol/L x 0.001 (convert mmol to
M) = 1.8 x 10-4 M/L.
I don't have a value for the Ksp
of CaCO3 at pH = 7.0; Holmes-Farley
supplies one for aragonite at pH = 8.2, natural seawater:
Ksp = 6.5 x 10-7
M2/L2
at 25°C. (Holmes-Farley, 2002).
So, supersaturation ≈
[2.3 x 10-2 M/L][1.8 x 10-4
M/L]/(6.5 x 10-7 M2/L2)
= 6.4. The CaCO3 supersaturation of
normal seawater (for aragonite) ≈
3. It is likely that this calculated value is high, as the
true Ksp for CaCO3
under pH 7.0 conditions is likely to be larger than the pH
8.2 value used.
If this analysis is correct, then why doesn't the CaCO3
just precipitate out at such a supersaturation? The answer
is, in fact, that it does, at least to some extent. Recall
from (1) above that about 20% of the salt mix is insoluble
at the end of the salt bucket's life, although no components
are insoluble if fresh salt is mixed to 35 ppt. It appears
that some of the soluble CaCO3 and,
presumably, MgCO3, precipitates out
during use. Because most of these ions stay in solution for
the salt bucket's entire life (cf. Figure 6),
however, the Dialyseas approach using a concentrated salt
solution appears to work. Why doesn't more of the Ca and CO3
precipitate out? The likely reason is tied to suppression
of the CaCO3 deposition rate. High
magnesium concentrations, as present here, interfere with
CaCO3 deposition by binding competitively
to the growing aragonite/calcite crystal face. This competition
retards the rate of CaCO3 crystal growth.
6) The ratios of measured components (Figure
8) change dramatically over time, and at almost no time
do these values correspond to natural saltwater values (given
adjacent to Figure 8). In contrast, the dialysis purification
process leaves these ratios relatively unchanged, and at values
similar to the natural saltwater ones (cf. Figure
5). These results raise the obvious question, is the Dialyseas
capable of maintaining an appropriate ionic composition over
time? I monitor the aquarium water's [Ca2+],
[Mg2+] and [alk] weekly,
and although these values fluctuate they do not exceed desirable
parameters under normal circumstances, the specific exceptions
detailed under II.7 Salt Delivery notwithstanding.
The [Ca2+] levels typically
fall within the 390 - 425 ppm range without supplementation
(i.e., CaCl2 addition), the alkalinity
spans about 3.5 - 4.0 meq/L, and the magnesium runs a little
high, as expected from the data presented in Figure
7, at 1500 - 1650 ppm. It is possible that this apparent
contradiction between the aquarium water values and the salt
bucket supernatant values can be resolved by noting that the
salt bucket's contents are disbursed into the aquarium water
in small increments, perhaps on the order of < 1 gpd (this
amount is not recorded by the Dialyseas). Compared to the
system water volume (~ 167 gallons), it is clear that the
effect of any one small increment of ionically unbalanced
concentrated salt solution will be negligible as a consequence
of dilution. Over time, the average ionic ratios must equal
the original amounts of components present, which are similar
to natural seawater. Thus, it is possible that the fluctuations
in salt component content are averaged out over the course
of the salt bucket's useful life.
Overall, these results demonstrate that the basic Dialyseas
design element of adding concentrated salt solution to regulate
the tank's salt content is not only feasible but can be implemented.
III. Summary and Conclusions
I started this article with a statement
about the claims Seavisions makes for the Dialyseas:
"The Seavisions website details Dialyseas' claimed benefits,
which include reducing the concentrations of undesirable solutes
(ammonium, phosphate, nitrate, etc.) to arbitrarily low levels,
and maintaining high water quality almost completely automatically
with little day-to-day input from the aquarist."
In my experience, the Dialyseas fulfills these claims.
I subtitled this analysis "Pros and Cons," and
in no way do I feel that I have been "conned by a pro."
After studying the system in operation for over 18 months,
I can find no evidence of deception or misinformation in Dialyseas'
description and marketing. The Seavisions Company offers a
quality product.
This system represents a novel approach to addressing the
water purification and water exchange requirements any aquarist
faces. By and large it performs as advertised, although the
problem with occasional oversalting should be noted. It has
proven to be a robust and reliable technology in my hands
and an asset to tank maintenance. Its automatic water change
function is its primary value to me for the reasons described
in Section I.6. Whereas other cheaper automated and semi-automated
pieces of equipment are available to perform water changes,
I am not aware of any that can actually increase the tank
water's salt content to bring it in line with a preset value.
These other systems can add premixed salt water, but that
operation, in and of itself, cannot raise the tank water's
salinity to a preset target level. One significant consequence
of this "concentrated salt solution approach" to
maintaining water quality is that the equivalent of 210 -
250 gallons (Seavisions estimate) of appropriately constituted
salt water can be packed into a volume no larger than 1 cubic
foot (see the salt bucket's picture in Figure 1). This space
savings has been a real benefit for me. The Dialyseas system
has distinct and unique advantages over other automated water
exchange methodologies on these two points.
I am less favorably inclined toward the value of its dialysis-based
purification capability, despite the fact that this purification
function appears to be the primary selling point advertised
by Seavisions. The data support the conclusion that the Dialyseas
can, in fact, deplete the aquarium water of undesired contaminants
without causing ionic imbalances in other desirable water
components. However, the concentrations of undesirable metabolites
(ammonium, nitrate, nitrite, phosphate, etc.) that it removes
are typically so low in a mature reef tank under standard
operating conditions that the extra purification by the dialysis
process seems, perhaps, to be overkill. On the other hand,
the concurrent removal of desirable solutes, in particular
calcium, seems like a big downside as it places added pressure
on the calcium input devices (Dialyseas concentrated salt
solution, Nilsen reactor, calcium reactor, others?) to keep
up.
In the final analysis, of course, the question of whether
the Dialyseas is worth the money is a question that can be
answered only by each individual aquarist according to his/her
own standards of expense vs. value. I hope that this analysis
has provided some useful guidance for those contemplating
this question.
Acknowledgments: Funding for this study from the State
of Pennsylvania and E. I. du Pont de Nemours and Company is
gratefully acknowledged. In addition, thanks to Randy Holmes-Farley
for a critical reading of this manuscript and many helpful
suggestions.
|

