|
All
sorts of odd creatures appear in reef aquaria from time to
time. Of course, by most non-reefkeeping standards, most of
the animals that aquarists keep are strange or unusual. After
all, people live on land and are most familiar with the organisms
that they encounter on a daily basis: dogs, cats, birds, and
houseflies; not reef aquarium animals. A coral reef is a vastly
different world from the one that most people are used to,
and it goes without saying that many of the animals from that
world are different to the point of being weird and unusual.
I think the strangest of all of these animals, however, are
those that biologists call echinoderms: the sea stars, sea
urchins, sea cucumbers and their relatives.
Echinoderms are highly complex animals
containing organ systems that are unique, unlike anything
seen in any other animals. They are typically animals with
no front end, no back end, no head, and no brain (Hyman, 1955;
Kozloff, 1990; Harrison and Chia, 1994; Ruppert et al.,
2003). They also are the ecologically dominant animals in
most marine ecosystems, including many coral reefs; without
their presence these environments become vastly different
places from how we normally think of them (Paine, 1966; 1974;
Sammarco, 1980; Paine and Levin, 1981; Birkeland and Randall.
1981; Glynn, 1985; De Ruyter van Steveninck, and Bak, 1986;
Glynn and Krupp, 1986a,b; Wallace, et al., 1986; Sano
et al., 1987; Faure, 1989; Walbran, et al.,
1989; Andrew, 1991; Cameron, et al. 1991a.b; Coyer,
et al. 1993; McClanahan and Mutere, 1994; Chess, et
al, 1997; Peterson et al., 2000; Carriero and McClanahan,
2001; Edmunds and Carpenter. 2001; Knowlton, 2001; Phinney
et al., 2001; Williams and Polunin, 2001; Barnes, et
al., 2002). Finally, many of them show no evidence of
the aging process. Unless attacked by some disease, eaten
by a predator, or killed by some environmental disaster, they
have the potential to live indefinitely. Old echinoderms may
be very old, indeed.
Given all of the strangeness attributable
to these animals, and their popularity in aquaria, I thought
I would devote several columns to the echinoderms; animals
I think are just about the neatest animals we can keep in
our aquaria. My last column
about any echinoderms was in the November, 2003, issue
of Reefkeeping and dealt with sea urchins. The present
series of columns will examine the group as a whole. This
first column will be a discussion of some basic echinoderm
properties, including their larvae. I will discuss other aspects
of their biology and the adult forms starting with next month's
column.
No Front, No Back, It Is All The Same To Them
When
we look at the world around us, one of the things we do is
automatically classify what we see. The need to pigeon-hole
things appears to be deep-seated in our species. Something
either belongs to a group or it doesn't; there is no halfway
house in our classification schemes. There are many ways to
classify the world around us, and various types of scientists
are actively pursuing new and interesting ways to do this.
For the average person, however, probably the most commonly
used scheme of classification is exemplified by the basic
question, "Is it animal, vegetable or mineral?"
Using such a question presents the person with a series of
choices: "Is the item, or has it been, living (animal
or vegetable) or is it non-living (mineral)?" Then after
that, presuming it is or was once alive, the question becomes,
"Is it an animal or is it a plant?" This is pretty
straightforward, although it begs the question for several
groups of living things that are neither plant nor animal.
Nonetheless, on a basic level, this question is pretty easily
answered for most of the items we are likely to encounter
in our daily lives.
If we decide that something is an animal,
what exactly does that mean to us? On a fundamental scientific
level, an animal is an organism made of more than one cell
that cannot produce food by photosynthesis. Such a distinction
would eliminate plants from consideration, but not the fungi.
To distinguish fungi from animals we need to add the criterion
that the animal's cells do not have rigid cell walls. Plants
typically have cell walls made of cellulose, and fungi often
have cell walls made of chitin, a different sugar polymer.
Animal cells typically lack cell walls altogether, having
just a simple membrane separating the external world from
the inside of the cell. Such a series of distinctions is well
and good, but is not something a non-scientist would likely
think of. What matters to most people is that animals are
mobile organisms that must feed to survive. Since most plants
and fungi don't wander around looking for a snack, these criteria
work pretty well, particularly in terrestrial environments.
When we look at the animals around us on
land or in the air, some other things are obvious criteria
for "animalness." The animals we are familiar with
have a front and back end, and along with these structures
they have a left and right side. This means that if we examine
one of these organisms, it can be divided into two halves
that are mirror images of one another by cutting it through
the body only on a plane that runs from the center of the
back to the center of the bottom surface. The property of
having two halves that are symmetrical about the body midline
is what is called "bilateral symmetry" and it characterizes
most animals. No animal is perfectly bilaterally symmetrical;
small imperfections or large deviations from such perfections
are relatively common; still it is, on a gross level, a generalized
characteristic that works for virtually all terrestrial or
airborne animals.
In marine and some fresh-water environments,
however, plenty of animals lack bilateral symmetry. Such animals
simply don't have a front, back, or two distinct sides. Perhaps
the best examples of such animals are sponges, many of which
lack any sort of symmetry at all. Possibly this lack of defined
body form was a factor preventing their recognition as animals.
It wasn't until the latter half of the eighteenth century
that it became widely accepted among the naturalists of the
time that sponges were, indeed, animals (Hyman, 1940). Other
animals such as corals, sea anemones, and their near and distant
relatives have what can be called radial symmetry. They have
no head or tail, but instead have a body that is fundamentally
a cylinder. The mouth, surrounded by one or more rings of
tentacles, is situated in the center of one end of the cylinder.
This orientation means that the animal can be divided into
two equal halves by cutting through it on a plane that passes
along any radius of the disk that makes up the mouth, or oral,
end of the animal. Radial symmetry is characteristic of organisms
that either don't move or move slowly. Many plants, for example,
have radial symmetry, and that tends to influence how all
organisms with radial symmetry have been viewed. The fact
that many people have trouble realizing that radial animals
such as sea anemones are animals is reflected in their name.
Anemones are plants, sea anemones are not, yet they are often
called "flower animals." "Animalness,"
it seems to many folks, requires bilateral symmetry.
 |
 |
Figure 1. Left: This handsome fellow is a specimen
of the great sculpin, Myoxocephalus polyacanthocephalus.
As with all fish, it is bilaterally symmetrical and can be
divided into two equal halves only by a plane running vertically
along the midline of the body. Right: This small, unidentified
hydrozoan is almost perfectly radially symmetrical. It may
be divided into two equal halves by a plane running lengthwise
down the body as long as that plane divides the oral disk
and tentacles evenly.
Radial symmetry is not particularly rare
among animals, but it is found in only four of the more than
forty major distinct animal groups called phyla. These are:
-
The Porifera, or sponges, of which many species have
fundamentally cylindrical bodies;
-
The Cnidaria, or corals, sea anemones, hydroids and jellyfishes,
of which virtually all species show a basic radial symmetry;
-
The Ctenophora, or comb-jellies, many species of which
are relatively close to being radially symmetrical; and
-
The Echinodermata, or the sea stars, sea urchins, feather
stars, and sea cucumbers, all of which show a derived
and somewhat imperfect radiality resulting from the drastic
metamorphosis of a bilateral larva.
Radially symmetrical animals typically
live as ocean bottom-dwelling animals that are sessile or
relatively slow moving, or pelagic animals. Although they
may be highly predatory, and may even have photoreceptors,
they do not generally hunt their prey visually. Instead, they
may follow scent trails to prey or, like most sea stars, they
may simply move around randomly until they encounter an acceptable
food item.
Similarities And Differences
I
find the changes that occur in animal bodies, either as a
result of evolution or as result of growth, exceptionally
fascinating. In some respects this probably accounts for my
love of the echinoderms, and particularly the group referred
to as the Holothuroidea, or sea cucumbers, which undergo the
most drastic changes of all. Recent genetic research focusing
upon structural similarities in the genetic codes of animal
groups has confirmed that one of the three great branches
of the animal kingdom consists of the Cnidaria, the Echinodermata,
the Chordata (animals such as fishes, birds, and humans) and
a couple of smaller related groups (Field, et al. 1988;
Smith, 1992; Turbeville, et al. 1994; Wada and Satoh,
1994; Jefferies, et al. 1996; Lacalli, 1996; Adoutte,
et al. 2000; Baldauf, et al. 2000; Jenner, 2000).
In these groups, the changes seen from one to the next may
provide a window into the distant past, showing some of the
evolutionary changes that occurred during the history of life.
Additionally, the conservation of some vital genetic traits
within these groups may appear as similar properties in what
are now vastly different types of animals (Strathmann and
Eernisse, 1994; Sprinkle and Guensburg, 1995; Daly, 1996;
Williams, 1996).
Although it is hard to imagine any two
animal groups that could be more dissimilar, echinoderms and
chordates share some fundamental biological properties. Many
of the basic biochemical pathways and properties of our cells
are the same as those found in echinoderms, but are decidedly
different from those found in the rest of the animal kingdom.
As an example, the chemical chitin, commonly used as structural
material throughout the animal kingdom, is totally lacking
in the echinoderm and chordate lineage. Similarly, many basic
structural similarities at the cellular level exist that link
these groups. As an example of this, the photoreceptors found
in echinoderms and the rod and cone cells that are the photoreceptors
of the vertebrate eye are ultimately derived from the modification
of the cilium of a ciliated cell. The photoreceptors throughout
most of the rest of the animal kingdom do not have a cilium
as their basis, being derived instead from a different cellular
structure called a microvillar surface. Finally, the early
embryology of chordates and echinoderms is strikingly similar
and, again, unlike that found throughout most of the animal
kingdom (Ruppert, et al. 2003).
The early embryology of the echinoderms has been studied
in detail for more than 150 years, and it is still yielding
information of value and interest. The study of animals such
as sea urchins and sea cucumbers has been undertaken, in no
small part, as a way to study an analogue of early human development.
For at least the first few cell divisions, animals such as
sea urchins and humans undergo fairly similar development,
which can be studied in detail without the potential ethical
problems that arise during the study of early human embryology.
As a result of all this research, we have a pretty good understanding
of the basic development of most types of echinoderms (Strathmann,
1987; Ruppert, et al. 2003).
How Do We Get There From Here?
One of the vexing
problems of biology is, "How does a multicellular organism
develop from a single-celled egg?" The control of this
developmental process has been a focus of much research, both
applied and basic, for most of the last century, and remains
so today. Literally hundreds of millions of dollars are being
spent annually on various facets of this question to elucidate
its important medical aspects. From the perspective of a marine
aquarist, however, probably the most interesting part of the
question remains, "How does the organism develop from
a fertilized egg, or zygote, into a functional, living animal
that can maintain itself in the environment?"
Biologists refer to this process as "embryological development,"
often shortened to just "development." Once an organism
has reached the stage of a small juvenile, further development
into a functional adult is pretty straightforward; often it
is simply growth, with the final development being the onset
of sexual maturity. This is, from the organism's view, of
course, a very big deal, but it is often easy to study and
understand. The organism's initial development, however, requires
significant and drastic changes in its morphology. These changes
are often much more difficult to get a good handle on, and
as such, have become much more interesting to study. What
happens in these early life stages of echinoderms has been
well studied, and is pretty well-known, at least on a gross
scale. However, that doesn't make it any less bizarre.
Echinoderms are great animals to use to study development.
They are almost all broadcast spawning animals, with fertilization
occurring in the sea. Consequently, they don't have egg shells
that need to be removed, and their developmental stages can
be followed in a container of sea water (but see Strathmann,
1987 for details). One characteristic of the cnidarian-echinoderm-chordate
branch of the animal kingdom is the production of eggs that
divide radially. To go from a single-celled zygote to a multicellular
animal, the one cell that is the zygote needs to subdivide
itself repeatedly. This repeated cellular division results
in an embryo with many cells, but with each cell smaller than
the one that divided to form it. Until the developing organism
can begin to feed, all the energy and raw materials for this
cell division must come from materials stored in the eggs.
Most of these stored materials are, of course, yolk.
When an echinoderm zygote divides for the first time, it
forms two identically appearing, equally-sized, cells. At
this stage, it is not a zygote anymore; it is now referred
to as "an embryo at the two-celled stage." These
two cells divide synchronously resulting in a four-celled
embryo. All of these cells are equal in size and when viewed
from above, they exhibit radial symmetry. So, at this stage
of its life, the echinoderm embryo is considered to show a
fundamental primary radial symmetry. It is worth noting that
most of the animal kingdom does not develop in this manner.
Mollusks, annelid worms, and many other animals have four-celled
embryos in which each cell is different in size from all others.
Arthropods and related animals have yet a third type of development
characterized by incomplete cellular division of an embryo
constrained within an eggshell.
Figure 2. Early cell division in a sea urchin, Arbacia
punctulata. The cells are not perfect spheres because
the fertilization membrane that surrounds the developing embryo
constrains its shape. Left: Undivided zygote. Middle:
Two-celled stage. Right: Four-celled stage.
The third cell division is also synchronous, but the plane
of cell division is oriented at right angles to the previous
divisions. In the idealized echinoderm embryo, this results
in eight cells arranged in two quartets with the third plane
of cell division at the equator. In practice, in most embryos
at this stage, four of the cells are slightly larger than
the other four. Subsequent to this stage, cell division continues
to be synchronous. After seven divisions, the embryo has 128
cells, and generally is in the form of a hollow sphere called
a "blastula."
Blastulae
are often ciliated and move through the water as small rotating
spheres.
At about this time the synchrony of cell division begins
to break down, and the number of cells becomes difficult to
calculate or count. Generally, at some time when the embryo
has between 128 and 256 cells, a dimple-like depression begins
to form at one end of the embryo. Through further cellular
division, the dimple becomes a pit and the pit becomes a tube.
The tube extends into the cavity within the embryo. This tube
is the developing gut. As the gut develops, the embryo appears
as a sphere with a hole in it. That hole is called a blastopore,
and is the opening of the tube on the surface. At this stage
of development, the developing embryo, called a "gastrula,"
has the same basic structure exhibited by a developing cnidarian
polyp, prior to the formation of tentacles. The embryo may
be visualized as a small cylindrical organism, with one tissue
layer on the outside of the body, another tissue layer lining
the gut, and with only one opening to the gut. This is exactly
the same fundamental architecture that is found in cnidarian
animals such as corals. At this stage of development the embryo
is still primarily radially symmetrical, but this type of
symmetry will disappear shortly (Strathmann, 1987; Young,
et al., 2001; Ruppert et al., 2003).
Further cell division results in the internal gut tube growing
until it contacts the far wall of the embryo. It grows to
and fuses with the far wall and an opening occurs in the wall,
resulting in a hollow tube extending through the embryo. This
tube is, of course, the gut of the small developing animal.
The second opening becomes the mouth in all echinoderms while
the first opening of the gut, the blastopore, becomes the
anus. Shortly after the gut becomes open at both ends, further
development occurs rendering it functional. At this stage,
the embryo is considered to be a larva, and is a feeding,
growing, and functional animal. Further development is generally
considered to be larval development, not embryonic development,
although these terms are not rigidly used. Concurrently, with
the development of the gut, internal structures are starting
to develop, and the animal ceases to be radial, and becomes
an elongated bilateral mobile consumer of phytoplankton.
All of the above processes happen quite rapidly. It is not
uncommon for an echinoderm embryo to go from a single cell
to a feeding animal within 48 hours. IMAGINE! What must occur
to convert a single cell with a relatively featureless interior
to a feeding animal in two days? The timing of what genes
turn on and off and the resultant changes that occur are simply
mind- boggling. It has been estimated that humans will create
simple life forms in culture vessels within a decade, possibly
much sooner. Such forms will mimic bacteria in their structure.
It will be a very long time, however, before we can turn a
single cell into a functional animal.
Swimming Sea Urchins And Other Prickly Critters
Young
larvae are simple animals, but they are functional animals
that must do all of the things other animals must do. They
have to eat, excrete, move, sense the environment, and avoid
being eaten. We used to think that the one characteristic
separating larvae from adult animals was that larvae didn't
reproduce. We now know better; it appears that many, if not
most, echinoderm larvae may be capable of at least asexual
reproduction (Vickery and McClintock, 1998; 2000; Eaves and
Palmer, 2003). Given that at many times of the year, the number
of larvae may exceed the number of adults by a significant
number, the fact that these larvae may be reproductive rather
turns the idea of species on its head for these animals. Perhaps
we should consider the so-called larva as the definitive stage,
with the so-called adult existing solely to produce more larvae,
rather than considering the larva as a stage to disseminate
adults. Although this seems like a semantics problem, it really
isn't. The larvae are subject to natural selection and evolutionary
pressures just as the adults are, and we really don't know
the relative contributions to either stage in the overall
life cycle that is the "species" in these forms.
It really is apparent that we have to consider
these animals as "life cycles" rather than as a
definitive final stage at any part of that cycle. While we
can readily identify most adult echinoderms, this is simply
a result of their being large and evident animals. Many of
the larvae are equally identifiable, once we know what characteristics
to look for. The whole cycle is broken if any part of it dies,
and that break is no more final if the death occurs in the
adult or larval phase.
Figure 3. Some bilateral echinoderm
larvae. Left: A young sea urchin larva or echinopluteus.
The internal skeletal rods are clearly visible. Middle:
An older sea urchin larva. This larva has been feeding on
green phytoplankton which is visible in the gut. Right:
A brittle star larva called an ophiopluteus. All of these
larvae move through water catching food, and they move in
the direction of the tips of the arms or, in other words,
the apex of the triangular body is the posterior end.
All echinoderms have bilateral swimming
larvae, and it appears that the most basic forms in many groups
are feeding larvae. A number of groups have non-feeding larvae,
but with the exception of the larvae of feather stars, these
forms appear to be descendents of feeding forms (Strathmann
and Eernisse, 1994; McEdward, 1995). Echinoderm larvae are
often relatively large as larvae go. The definitive larval
stage, the stage that will metamorphose into a juvenile, is
about a millimeter or more in length and some are much larger
(Strathmann, 1987; Young et al., 2001). As larvae go,
these are giants.
The one feature that is constant about
larvae is that they change, they grow and otherwise develop
new features; consequently, it is hard to discuss a "typical"
echinoderm larva. There really is no such animal. There are,
however, some common attributes and structures found throughout
all the larvae. While echinoderm adults are radially symmetrical,
the larvae are bilaterally symmetrical. They don't have a
head, but they do have a front end, and they have sides that
are mirror images of one another. Both internal organs and
external surfaces reflect this symmetry, at least in the early
forms. Echinoderm larvae are complex. The gut is regionated.
There is a mouth, esophagus, stomach, intestine and anus.
There are internal body cavities and tissue structures that
develop and surround the gut. There is a larval nervous system
that appears to be quite complex and sophisticated. Little
is known about the nervous system; it consists of exceptionally
small cells that are very difficult to see and work with,
but over the last few years it has been demonstrated just
how complex this system is (Nakajima, et al., 1993).
The larvae have various behavioral patterns and within their
scale of size some of them are quite able to avoid areas of
distasteful chemicals and predators while choosing to remain
in areas of high food concentrations. The larvae are capable
of selecting certain types of the unicellular algae they use
as food while rejecting other types. Generally, the larvae
have a discrete internal skeleton made of calcium carbonate
rods. This skeleton may be very complex, depending upon the
larvae, and the rods may even articulate with one another
resulting in moveable appendages. Finally, the change from
the larvae to the juveniles requires a complex metamorphosis.
This is unusual in that only a portion of the larva typically
undergoes the change into the juvenile form. While in most
cases, the remainder of the larva is consolidated into the
juvenile, in some cases, it appears the remainder may actually
be able to persist, perhaps giving rise to more juveniles.
First Cousins, A Quarter Of A Billion Years, Removed
The
group of animals referred to as the Phylum Echinodermata is
truly ancient. These animals were successful and dominant
within the seas that covered the world with the first blossoming
of large animals during the Paleozoic
Era that existed from about 525 to 225 million years ago.
Significantly more kinds of echinoderms were living during
those times than are now alive. As with all other kinds of
life, the echinoderms were dramatically reduced in number
and diversity during the "Great
Dying" that occurred at the end
of the Paleozoic period (Tasch, 1973). Over 95 percent
of all marine species went extinct during the extinction event
that marked the end of that period. However, the extinctions
were not evenly spread over all groups. This resulted in survivors
from only a few of the array of echinoderm types and this,
in turn, resulted in a confusing array of larvae with no intermediate
types. Many of the distinctive types of echinoderms present
in the Paleozoic Era went extinct at the end of that period,
and it is likely that many forms with intermediate larval
forms perished. Unlike the situation seen in the crustaceans
where there is an array of progressively more complicated
larval types, within the echinoderms the larvae from each
of the groups are complex. There are no "simple"
ones.
 |
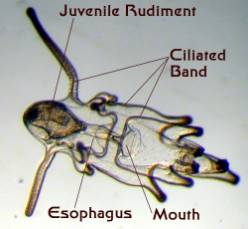 |
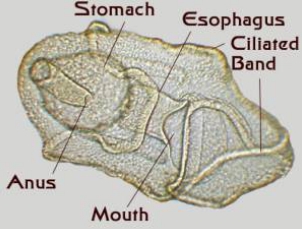
Figure 4. Starfish larvae. Left: An early larva
prior to the development of a juvenile rudiment.
Right: A late stage larva with a developing juvenile
inside of it. This larva was about an eighth of an inch long.
The larvae all tend to have some common
characteristics, but these are elaborated within each group
in some truly wonderful ways. In many ways, these animals
would make interesting aquarium animals, if only they were
large enough to see easily. Unfortunately, they are just on
the edge of easy viewing without a microscope.
Some
sea cucumbers and feather
stars develop larvae that basically barrel shaped. They
are surrounded by "barrel hoops" of cilia and these
little cylinders move though the water with surprising speed.
These larvae generally don't feed and some of them metamorphose
directly into juveniles. Sea urchins, sea stars, and brittle
stars have more elaborate larvae which often have elaborate
sets of appendages. In these larvae the locomotive force is
generated by ciliary bands that are arranged around the animal,
often in a very elaborate pattern. These bands of cilia also
collect the small unicellular algae upon which these larvae
feed. Sea
star larvae lack elaborate internal skeletons; however,
both brittle star and sea
urchin larvae have very elaborate skeletons (Strathmann,
1987; Young, et al., 2001; Ruppert, et al.,
2003).
And the Story Continues…
At
the end of the normal larval period, the juvenile begins to
develop as a rudiment inside the larva. This juvenile rudiment
may become quite large and well developed. When the rudiment
is about ready to exist on its own, the larva tends to swim
down near the bottom and search for an appropriate habitat.
During this period, it often touches the substrate, presumably
tasting the surface. If it finds an appropriate habitat, it
will often settle to the surface and in some groups even attach
to the surface. The juvenile rudiment will then tend to undergo
a drastic shape change; in some cases, it effectively turns
itself inside out. This results in a functional small sea
urchin or sea star that has given up its bilateral symmetry
and taken up its radially symmetrical bottom dwelling form.
If the larva doesn't find the appropriate habitat the metamorphosis
can be delayed for a while. If no appropriate habitat is found
within a short period, however, the larva and the juvenile
within it will typically perish; these animals generally do
not metamorphose into unacceptable habitats. For those animals
that do find the appropriate habitats, metamorphosis means
changing into radially symmetrical animals far different in
form from the larvae, but also far more familiar to reef aquarists.
The metamorphosis also means taking up residence in or on
the ocean bottom, and that is a tale that I will continue
in next month's column.
Figure 5. Late Stage Sea Urchin Larvae. Left:
A well-developed echinopluteus showing the beginning of the
juvenile rudiment beside the gut. Middle: The juvenile
rudiment is well developed in this larva. Right: This
larva is just about ready to metamorphose into a small sea
urchin. The radial nature of the juvenile rudiment is evident
and the juvenile locomotory organs, called tube feet, have
been formed. The larval body is being absorbed into the juvenile,
but it was still able to swim as this larva was collected
in the plankton. All of these larvae were about one tenth
of an inch long.
|