| Many
invertebrate animals have ways to capture prey or defend themselves
that result in an injection of a toxin into a target organism.
Aquarists need to be aware of such structures or behaviors for
two simple reasons: First, such an injection usually hurts.
Second, such an injection may, in rare situations, be lethal.
Either of these outcomes can really spoil one's whole afternoon,
but at least in the latter case, the pain although often severe,
is of limited duration.
Envenomation is the technical term for
an injection of toxin, and there are really quite a wide variety
of marine animals which have some method for doing this. Interestingly
enough, many of the most sought-after animals in the aquarium
hobby are capable of stinging some other animal. In this column,
I will discuss in some detail the envenomation apparatus of
some of the more commonly encountered stinging organisms,
and will also discuss in passing some of the other animals
that sting, even if they are rarely encountered in aquaria.
The animals that actively sting really seem to be concentrated
into two major groups: the Cnidarians, or corals, sea anemones,
and jellyfishes; and the Mollusks, such as some snails. Other
animals, such as fire worms and sea urchins, might have venom-laden
spines, but they are generally passive in their delivery of
the toxin. Some other animals, such as blue-ringed octopuses
and flower sea urchins, bite to inject a toxin. It may be
splitting hairs to some extent, but in this column I will
assume that a bite is not a sting. Here I define a sting to
be a puncture wound specifically designed to inject a venom
below the epidermis; in effect, a sting is a hypodermal injection
of venom.
For information on marine envenomations
of all sorts follow this link.
The Cnidarian Nematocyst, An Intraepidermal
Bomb
The corals, sea anemones, and jellyfishes
are grouped together by scientists, in large part, because
of their possession of a unique stinging apparatus. This structure
is called a nematocyst. Nematocysts are invisible to the unaided
eye, but are found by the millions in large cnidarians. Smaller
animals, of course, have fewer of them simply because they
have less surface area, and the number of nematocysts is dependant
upon the extent of surface area. With only few exceptions,
all cnidarians possess nematocysts, and those that lack them
are thought to be descended from ancestors that once had them.
What is this thing called
a nematocyst? Nematocysts are secretions of some peculiar
cells found in all cnidarians. Most people tend to think that
all cellular secretions are of a liquid or fluid nature, such
as mucus or perhaps a digestive enzyme. Actually, such materials
are simply solids dissolved in a fluid base, and there are
many potentially solid materials secreted by individual cells.
Such solid materials are often fluids which harden on contact
with air or water, such as the protein that constitutes the
tube of a feather duster worm. Other materials may be secreted
as crystalline solids such as the spicules of a sponge. Nematocysts
are proteinaceous capsules secreted in such a way as to have
an internal thread-like structure. The capsular wall is very
tough and resistant to deformation, yet it is permeable to
water. Additionally, the contents of the capsule are quite
concentrated. This means that there is a much higher relative
abundance of water outside the capsule than there is in it.
Such a disparity of water concentrations means that water
tends to flow into the capsule by osmosis. This osmotic flow
builds up a significant internal pressure in the capsules;
the pressure has been measured at an equivalent of over 2000
pounds per square inch. The protein coating the capsule prevents
the capsular contents from escaping.
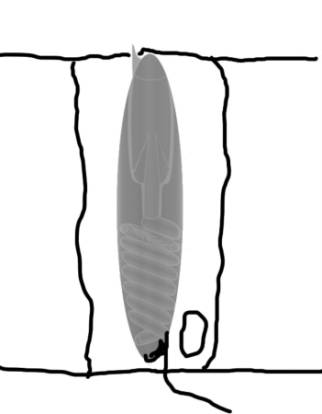 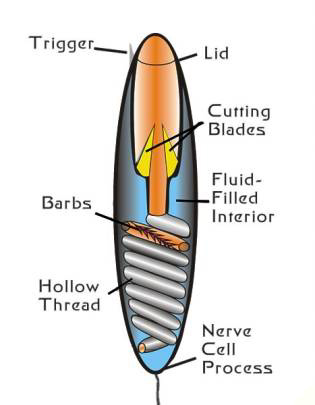 |
|
Figure 1. A diagram of an undischarged
nematocyst in a cell (left). A diagram of the structures
found in a nematocyst (right). Compare with Figure 2.
|
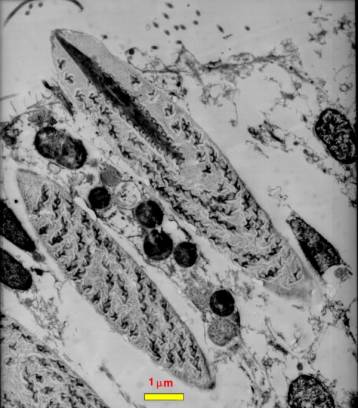 |
|
Figure 2. A transmission electron
micrograph of a section through a coral epidermal cell.
Three nematocysts are visible, one at the lower left
is only partially seen. Directly above it another nematocyst
is visible. This one has been sliced through tangentially
off of the midline of the nematocyst. Above this in
the center of the figure is a nematocyst cut through
the midline. Note the internal structures. Compare with
Figure 1 to identify them; the thread spiraled around
the inside of the nematocyst is particularly evident.
This photograph is copyright 2002 by Eric Borneman and
used with permission.
|
Under certain specific conditions, the
most exterior portion of the capsule may rupture, releasing
all of the internal contents. "Releasing" is much
too mild a word to describe this process, though. It is not
an exaggeration to refer to the contents as "exploding"
from the cell. When the capsular contents are blown out, the
internal thread is turned inside out and exits the capsule.
Generally, the tip of the thread is hollow and the capsular
contents will be sprayed from the tip of the thread.
It is impossible to watch the contents
of the capsules themselves as they exit the nematocyst. However,
the internal nematocyst thread may be filmed as it leaves
the capsule using ultrahigh-speed photography, and is by no
means easy to do. When it is done properly, however, a timed
record of nematocyst discharge is available. From one such
record, it was estimated that the tip of the nematocyst thread
is forced out of the capsule at the astounding acceleration
of 40,000g! Even though the tip of the thread is minute, with
such acceleration driving it, it can punch through almost
all biological surfaces, including some mollusk shells, arthropod
exoskeletons, and human skin.
What makes the action of nematocysts even
more "fun," if some happen to be triggered into
your tissue, is that the cellular contents discharged by the
nematocyst may be anything from toxins to digestive or lytic
enzymes. Most of these materials are proteins. As far as the
aquarist's body is concerned, these contents may do one of
three things:
|
•
|
First, if they are discharged
into thickened epidermis, such as on the palms of the
hands, the thread may be too short to penetrate the dead
epidermal layers, and the nematocyst discharges do nothing.
In these cases, the aquarist may be considered lucky. |
|
•
|
Second, if they are discharged
into areas where the skin is thin, for example the inside
of the forearm, they may cause pain and tissue damage.
As an example, one person I worked with had the tentacles
of a large fish-eating anemone from the Pacific Northwest
brush across the inside of her arms in a display aquarium
she was maintaining. The nematocysts left a trail of red
pustules that developed into open ulcerative lesions that
took about 2 months to heal. |
|
•
|
Third, in such cases where
the discharged proteins make it into the blood stream,
there is the possibility of an allergic reaction. The
materials discharged are foreign proteins, and their presence
in the body initiates an immune response. This can, in
some cases, develop into allergy, and further put the
person at risk to severe allergic reactions, such as anaphylactic
shock, if they get subsequently stung again. About 20
years ago, after a few months of working with sea anemones,
I developed an allergic reaction to sea anemone stings.
Consequently, I have to be quite careful around the sea
anemones in my tanks. |
Not all cnidarian nematocysts are dangerous,
and some of the time they will not be of concern to aquarists.
Nonetheless, many of the stony corals and most of the sea
anemones that aquarists maintain have the capability of stinging,
and in some cases this sting can be dangerous. Perhaps even
more dangerous than the initial sting, is the possibility
that that sting will inject foreign materials that will cause
sensitization to subsequent stings. This may occur with the
first sting, or it may never happen. However, if it does,
such as with people who are sensitive to bee stings, the second
sting may be lethal.
For descriptions and images of anemone
stings from intertidal and subtidal anemones in Britain follow
this link.
Here are some images
of the results of stings from a jellyfish called the sea wasp.
The Australian box jelly, Chironex fleckeri,
is responsible for more human fatalities than shark attacks.
Here is a link
to some information about it and some rather gruesome images.
The cnidarian nematocyst is a microscopic
stinging apparatus that functions much like a small bomb,
located in the tissue of the coral, jellyfish, or sea anemone.
When each individual nematocyst is detonated on contact with
the prey or some other organism, it sends a small amount of
toxin into that animal. Generally, the discharge of a single
nematocyst has very little effect; however, nematocysts don't
exist singly. They are found in groups or bunches, and each
may have several thousand nematocysts that all fire at once.
The density of nematocysts at places in the epidermis of cnidarians
may range upward to about 10,000 per mm (or about 6,000,000
per square inch).
Here are some links to information and
illustrations of nematocysts:
hypnea.botany.uwc.ac.za
www.users.totalise.co.uk
The toxins found in nematocysts vary, and
not all nematocysts inject toxins. However, when the cnidarians
are specialized to capture and kill fish, their toxins are
tailored to vertebrate physiology and will have some effect,
to a greater or lesser degree, on humans and can be dangerous.
All clownfish-hosting anemones will eat fish, as will some
of the larger corals. The nematocysts from these animals are
all potentially dangerous to aquarists, and the animals should
be treated with caution.
Here is a link
that discusses treatment for sea anemone and other cnidarian
stings.
Slimy Snail Superstingers...
One of the ways that can be used to measure
evolutionary success is to tabulate the number of species
within a group. Those groups with a lot of species have exploited
more ecological situations and habitats, and consequently
the groups are considered to be successful. The Molluscan
Class Gastropoda, or "the snails" is among the most
successful of all animal groups; depending upon the "authority"
chosen, there are an estimated 50,000 to 150,000 species of
snails. Consider that there are only about 3,500 mammal species,
or about 9,000 bird species, and it soon becomes apparent
that even though they move at a snail's pace, these slimy
animals have undergone an evolutionary diversification into
an enormous number of species.
Most snails make their way through the
world slowly crawling around on a broad foot rasping at food
they encounter with a feeding organ called a radula. This
radula can be thought of as a rough rasping tongue. In some
snails, the radula contains teeth which are hardened with
hematite and opal, and it can rasp through just about anything.
In many others, such as the "turbo and trochid"
grazers, the radula acts more like a leaf rake, sweeping diatoms
into the mouth. However, there is one very large and diverse
group of snails which have abandoned scraping their prey off
the rocks, and have gone into the business of spearing their
food with a hypodermic harpoon.
These are the snails in the group loosely
called the "toxoglossa." The name is derived from
the Greek roots "toxon" and "glossa"
and means "bow tongue" as it was at one time thought
they shot arrow-like teeth into their prey. Interestingly
enough, the Greek term "toxicos," meaning
"poison" derives from the same root, "toxon,"
as the ancient Greeks occasionally used poisoned arrows. One
might be tempted to think the name for the snails should mean
"poison-tongue," however, such a word would be "toxicoglossa,"
rather than "toxoglossa." In any case, the snails
don't shoot arrows at their prey, nevertheless, the name stuck
like an arrow into a bull's eye. Instead, they harpoon their
prey and kill it so rapidly that only one small group of animals,
the strombid conchs, has ever developed an escape response
to them. All of the rest of their prey, including fishes,
have no escape response to them, whatsoever. Neither do they
have any tolerance to the venom. If they get stung, they die.
It is worth remembering that for an escape response to evolve,
some potential prey must escape and live to reproduce. If
none do, then there will be no inherited response.
The venomous snails include, but are not
limited to, the cone snails, and in fact some 20,000 different
species have been put into the toxoglossan group, and only
about 600 of these are truly Cone snails, or snails in the
genus Conus. The others are put into several other
groups, and some of these also kill their prey by stinging
it, but none of these are dangerous to humans. Although the
Cone snails are the most well-known of the stinging snails
and are exclusively found in the tropics, the others are found
in all seas, including the tropics, the polar seas, and the
abyss.
For illustrations of venomous snails that are not cone snails,
follow this link.
Both the nematocyst of the coral and the
tooth of the snail are adapted to sting their prey. However,
the similarities end there. Nematocysts are found inside of
cells, while the venom and stinging apparatus of toxoglossan
snails is a quite complex organ system made up of several
different structures and organs:
|
•
|
The false mouth or rhynchostome;
a vertically oriented slit at the front of the head. From
the outside of the animal, it looks like the mouth, but
it isn't. It opens into the proboscis chamber. |
|
•
|
The proboscis chamber,
or rhyncodeum, is a cavity within the head of the snail
containing the mouth and retracted proboscis. |
|
•
|
The proboscis, made of
modified lips, forms a coaxial tube that surrounds the
true mouth. When fully expanded, it can extend out of
the rhynchostome and is often as long as the snail's shell. |
|
•
|
The mouth, which opens
internally inside the proboscis. |
|
•
|
The mouth, or buccal,
cavity is behind the mouth and contains the openings for
the radular sac, venom gland, and salivary glands. |
|
•
|
The radular sac contains
the modified radula which secretes the harpoon-like teeth.
|
|
•
|
The teeth are formed in
one part of the radular sac and stored like arrows in
a quiver in the other part. |
|
•
|
The venom gland opening
into the buccal cavity just behind the radular opening. |
|
•
|
The venom gland, which
is a long tube located in the blood cavity inside the
head. It terminates in the football shaped muscular bulb. |
All of these structures constitute
the venom apparatus of a toxoglossan snail.
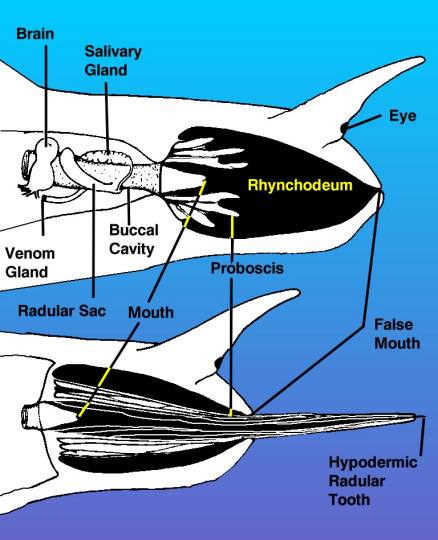 |
|
Figure 3. A diagram of the
head of a venomous snail, drawn from the right side,
showing the venom apparatus as if the tissues of the
right side of the head were transparent. The top view
shows the proboscis withdrawn inside of the rhynchodeum
or proboscis cavity. The drawing shows the proboscis
as if it were cut through, but remember it surrounds
the mouth as a tube. The bottom view shows the proboscis
extended with the hypodermic tooth held in the tip.
|
 |
|
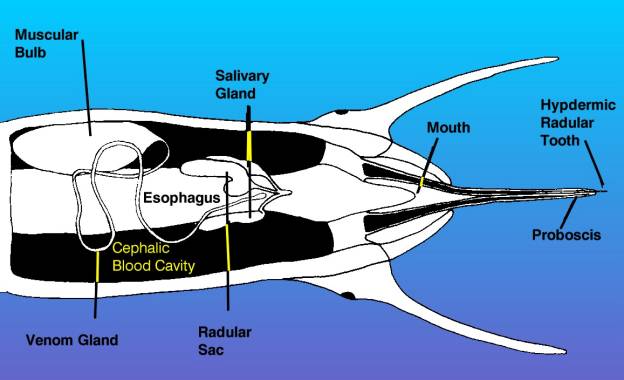
Figure 4. This is a diagrammatic
view of the head of a venomous snail, showing the proboscis
extended as in the bottom diagram of the preceding figure.
This view is from the bottom of the animal looking up.
The venom apparatus, consisting of the muscular bulb
and venom gland is visible, as are the radula and salivary
glands. Other structures have been omitted for clarity.
|
 |
|
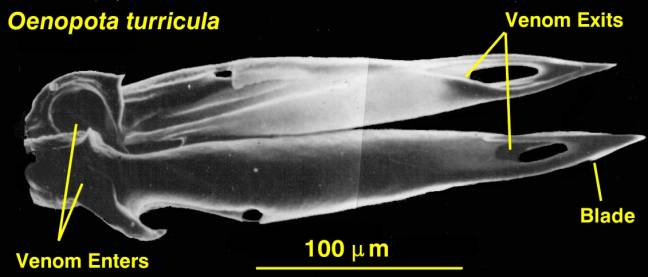
Figure 5. The hypodermic tooth
of a venomous snail from the N. E. Pacific, Oenopota
turricula. The teeth of the tropical Conus
are essentially the same in basic structure, but are
about 10 times as large.
|
When one of these snails finds something
it wishes to sting, a tooth is moved from the radular sac
into the buccal cavity, where the barbed and bladed end is
moved to a forward position. The tooth is then moved forward
out of the mouth until the "hilt" of the spear-like
tooth is gripped by a muscular sphincter at the tip of the
proboscis. While this is happening, the venom gland secretes
enough venom to backfill the buccal cavity and proboscis.
Muscular contractions in the proboscis wall extend the proboscis
through the false mouth, and for some distance outside the
animal. The proboscis aims for the prey, with the tooth held
in the tip, probably guided by sensory input from the tip
of the proboscis as well as by sense organs near the mouth.
When the prey is contacted, the tooth is rammed into the prey
and the muscular bulb contracts, forcing venom into the prey.
In some cases the force of envenomation is sufficient to blow
venom completely through the prey! This venom is unbelievable
in the way it acts. In the case of those Cone snails that
spear fish, the fish dies effectively immediately, although
it may twitch for a while. The snail then crawls up and engulfs
the prey. I hope the reader will take the time to follow a
couple of the links for a look at the movies of Conus
spearing prey.
Most of these venomous snails are specialized
predators that eat only one or a few species of polychaete
("bristle") worms. However, some species prey upon
other snails, and only about a dozen species of Conus
(out of about 600 total species of Conus) eat fish.
In general, the sting of these worm and snail eaters is harmless,
or at worst irritating, although repeated stings could lead
to sensitization and allergic reactions, I suppose. The fish
eating Cone snails, on the other hand, pack a venom that is
amazingly lethal to all vertebrates, not just fish.
This fish-killing venom, called conotoxin,
varies in its composition from Conus species to Conus
species. All of these venoms, however, share some general
properties. They are fast acting, and are a mixture of several
different chemicals. Conotoxins are basically neurotoxins,
and kill by disabling the prey's nervous system. Most animal
neurotoxins are limited in their action; they typically disable
only one part of nervous function. Generally, this is enough
to rapidly immobilize the prey. The predator, often a snake,
can follow the track of the prey if it has escaped, find it,
and eat it.
Conotoxins are different. Effectively,
they are multiphasic and kill nerves in every single different
way that they can be killed. Animals stung by Cone snails
don't usually go anywhere. This is good for the snail, as
Conus has been described as being the slowest moving
of all snails, although there is some doubt about that. Prey
that die even a short distance from the snail would tend to
be lost to the predator, so apparently natural selection has
favored the development of these very potent venoms.
What really makes the venom and these snails
interesting, of course, is that humans have died as a result
of Conus stings. Just how many people have been killed
by the snail is not known, but estimates range upwards of
50, or so, in the twentieth century.
For information on human fatalities see
this link.
The problem with determining whether or
not the person has died of the snail's sting, is that it is
not immediately lethal. Humans are just a tad bit bigger than
your standard goby, and so the action of the venom generally
takes a while; say ten minutes to several hours. Death may
be due to cardiac arrest and may mimic a heart attack. It
is conceivable that many more human fatalities have occurred
and were mis-identified as being due to some other cause.
In cases of known stings by fish-eating
Cone snails, the majority of the human victims have died.
Considering the very small amounts of venom being injected,
this venom is one of the most lethal animal venoms known.
Interestingly enough, the very lethality makes it attractive
as a drug to treat some serious disorders, and a very significant
amount of research is presently being done to modify the components
of the venom so that it may be used to treat diseases.
For drawings of fish-eating Cones, including
one eating fish, follow these links:
www.manandmollusc.net
www.starfish.ch
For information on the treatment of Conus
stings, see these links:
www.pharmacology.unimelb.edu.au
www.emedicine.com
For information on Conotoxins and all things
about Conus, including links to identification pages
and movies of the snails killing fish, follow this link.
Of course, now that I have raised the potential
spectre of aquarist-killing snails lurking in reef aquaria,
I should note that the likelihood of this is vanishingly small.
Of course, if it does occur and someone gets stung, I suppose
the victim would take small comfort in those odds.
How To Identify a Potentially Deadly Snail
As any regular visitor to my "Ask
Dr. Ron" forum knows, there are a lot of different snail
species, and they are generally NOT easy to identify to that
level. However, given that aquarists don't need to identify
these particular animals to species, but rather just need
to know enough to avoid them, the problem is simplified considerably.
Only Cone snails are potentially dangerous:
all non-Cone snails are safe. So, if you can identify and
remove Cone snails from your system, you won't have any worries.
Here are some hints to help you identify a Cone snail.
|
•
|
Conus species are
smooth-sided snails whose shell shape looks like a smooth
ice-cream cone. There are no ridges and no sculpturing
on the shell. It will typically be smooth, although there
may be some narrow grooved lines in the shell. |
|
•
|
All snails have an aperture,
or opening, to the shell. This opening reaches one end
(the front) of the shell. The opposite end is called the
"spire" and terminates in the shell "apex."
In many snails, the spire is elongated and tall. In the
Cone snails, it is not. The spire is compressed and low,
often flat. The shell really does look like a cone. |
|
•
|
The shell aperture is
typically slit shaped. It is not round, nor is it oval.
In the dangerous species of Conus, the front part
of the slit may be flared a bit, so that the animal can
ingest fish. |
|
•
|
Color is not a character
used to discriminate these species. Some species of Cone
snails come in almost all colors, and if you have one
that has been introduced with live rock, it is likely
to be covered with coralline algae or some other material.
Others, mostly sand-dwelling species, are often brightly
and beautifully colored, and these are often the dangerous
ones. |
If you find a snail that has all of these
characteristics - or maybe even just one or two of them -
and you remove and dispose of them, you won't have any trouble
with stinging snails. If you do think you have one of these
animals in your system, do not reach in and grab it bare handed!
Use some tongs to grasp it and remove.
To verify your identification, use this
link
and then follow the internal links to Conus pictures
and identification guides.
Now, it is also time for the disclaimer.
Cone snails of any sort are very unlikely to appear in any
aquaria. They are simply not collected to be sold to hobbyists.
The only way they are likely to find their way into an aquarium
is by being found as hitchhikers on live rock. Additionally,
while there are about 600 species of Cone snails, only about
a dozen are dangerous. The odds of finding any Cone snail
at all are pretty slim, the odds of finding a dangerous one
are really pretty small, and nothing to lose sleep about.
These two groups of animals, the toxoglossan
snails and the cnidarians, are at the opposite ends of the
spectrum of stinging animals. Structurally, they are very
different, yet both groups have similarities. Neither corals
nor Cone snails move much to find their prey, and both are
adapted to use rapid acting chemicals to kill their prey.
The cnidarians inject their prey with millions of tiny stings,
each containing a minute amount of venom. The toxoglossans
inject their prey with one or a few stings containing a small
amount of highly toxic venom. In each case, this feeding mode
has proven to be very successful. Cnidarians are common members
of benthic marine communities throughout the world, and toxoglossan
snails, which originated in the Mesozoic period, are rapidly
speciating and are amongst the most successful groups of snails.
|

