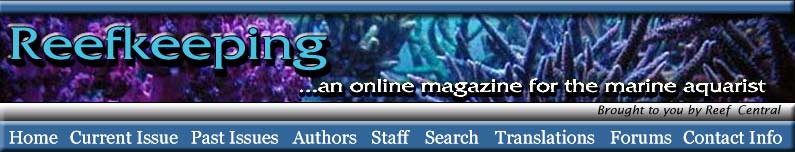
 |
 |
The industry of coral propagation seems
to be reaching a state of enlightenment where the knowledge
of fundamental procedures for the simple division of reef
invertebrates is becoming time tested and even commonplace.
It is wonderful to see so many corals in captivity that once
were thought to be impossible to keep alive not so long ago
now routinely pruned like shrubbery. In gross terms, the captive
propagation of coral may be categorized by the action of the
event: induced passively, naturally occurring or imposed.
Passive induction would include strategies of division
that neither result in the immediate production of a free-living
clone, nor will they necessarily occur unassisted. Rather,
such techniques are methods for spurring budding through fission.
Some examples of induced passive division include slicing
or notching the periphery of the stolon mat of hardy soft
corals such as Star Polyp (Pachyclavularia) or nicking
the exposed and illuminated stalk of a leaning (or forcibly
tilted) stalk of an Alcyoniid, which often spurs the budding
growth of beautiful multi-stalked colonies. Natural strategies
of captive coral propagation occur with various manipulations
and/or imitations of natural dynamics of the reef environment
and are being seen with increasing regularity. They are indeed
some of the most interesting events to behold and the subject
of another discussion altogether. Indeed, harnessed natural
reproductive events like planulae harvest are the future of
our trade.
For more than a few years, however, the
most common coral propagation technique has been the imposed
fragmentation of soft and stony reef corals through cutting,
breaking or sawing. By definition, these are deliberate actions
taken to asexually propagate a coral and produce divisions
that are free-living clones of the parent/donor. They are
the fastest and most popular way to farm corals to date, and
they are the foundation of our cottage industry. Indeed, imposed
fragmentary techniques will likely dominate coral farming
until larval rearing techniques are refined.
For new aquarists, the act of cutting into
living tissue to propagate an animal is brave and formidable.
Nevertheless, it is important for all aquarists to remember
that while useful generalizations can be made about a family
or even specific genera of coral, there are indeed exceptions
where a specimen does not conform to successful procedures
for the family/genera as a whole. A popular example would
be the "sensitive" colored and/or heavily mucous
Alcyoniids nestled in a family that mostly includes overwhelmingly
hardy "Leather" corals. What I mean by the categorical
distinction of "mucous" corals are species that
are easily and conspicuously stimulated to produce mucus,
which becomes readily apparent to an aquarist. To improve
rates of success for attempts at coral propagation, I would
advise aquarists to find experienced fellow enthusiasts locally,
in aquarium societies or online, to reassure them about unfamiliar
efforts. It is natural and appropriate for new coral farmers
to be nervous and conservative about unfamiliar imposed techniques
on coral. Rest assured, though, that hardy and established
corals that are suitable for propagation are likely to be
durable when propagated. In many years of travel, and with
my experience as a coral farmer, I have seen some extraordinary
events and testimonials to the survivability of corals. I
have personally run Trachyphyllia through saws with
amazing success and have watched aquarists mince whole Sarcophyton
individuals into 1/4 and 1/2" bits which were then thrown
into a rubble trough to produce many hundreds of daughter
colonies from the single event. Life in the ocean is dynamic
and harsh for many corals. As aquarists, we must put forth
our best effort to maintain optimum conditions in aquarium
systems with the understanding that our charges may be more
durable than we readily give them credit.
When setting to the task of cutting a soft
reef invertebrate or sawing a stony coral, one must first
determine the viability of the specimen and the optimal divisive
technique. While many reef invertebrates will tolerate fragmentation
so favorably as to not only yield clones, but also express
increased growth from such pruning, other corals may suffer
measurably instead. It can happen to the extent that the parent
colonies are not only inhibited from lending future fragments,
but also run the risk of succumbing wholly to infection. One
of the first distinctions to be made on a viable candidate
for fragmentation is the extent to which the animal might
be considered to be mucus producing. Most heavily mucous species
are categorically worse subjects for techniques of imposed
fragmentation, and are usually more safely farmed with slower
constrictive techniques (in octocorals) or passive induced
strategies (with scleractinians... especially larger polyped
species) with the glaring exception of Acropora, which
are quite tolerant of fragmentation. A familiar example of
this distinction to aquarists can be drawn in the comparison
of leathery Alcyoniids (most Lobophytum, Sarcophyton
and Sinularia) to heavily mucous Alcyoniids like Cladiella,
Klyxum (Colt), and other "colored" leathers
in this family. By "colored" leathers, I mean to
convey a categorical generalization that many of the bright
yellow and green "finger" and "mushroom/toadstool"
corals are often more sensitive to handling (shipping, damage,
propagation techniques, etc.) and may fairly be treated with
the same consideration we give to so-called mucous corals.
On the contrary, a common Sarcophyton is quite close
to "indestructible" with regard for propagation
techniques. One of my favorite non-traditional farming procedures
for the maximum production of divisions with a parent Sarcophyton
that one really doesn't want to maul aesthetically is the
"Doughnut" technique. The targeted Sarcophyton
will have its polyps "waved down," and is then removed
to a prepared cutting board for a brief procedure out of water.
The specimen is then inverted upon its "crown" while
a 1/2" to 1" doughnut of tissue is cut away from
the entire periphery of the "crown" (capitulum).
After an appropriate run through heated water baths (temperature
adjusted to match system water) to purge any mucus, the parent
is to be returned to the display in exactly the same position
from which it was taken. Strong and appropriate water flow
on the healing parent will help to prevent infection and form
a callus on the incised wound on the "crown," perhaps
in mere days. In as little as two weeks, the assault may hardly
be evident at all on the parent. The incised doughnut of tissue
can be chopped into equal sized pieces, tossed into a rubble
trough for growout, or be stitched/glued individually. The
creative sort might even let the ring settle and grow out
wholly undisturbed in a most unique shape! By comparison with
a mucous animal, nearly all such maneuvers described above
would not be readily possible like with a common branching
Colt coral (Klyxum), for example. Such a coral will
barely tolerate the simple cutting of branches, which are
then notoriously difficult and slow to settle and attach to
a new hard substrate. Tip: don't waste your time on
super glue or natural settlement here... use a single quick
stitch (use gloved hands or tweezers to reduce mucus stimulation)
through the base of the stalk of the division and tie it off
to a hard substrate. After several weeks, or when the animal
is apparently secure, snip and extract the discreet nylon
stitch. For propagating such mucous species, slower constrictive
processes like the use of plastic cable ties initiate very
little or no mucus production and are an arguably better method
of propagation here than cutting. The division often attaches
conveniently to the "handle" of the constrictive
tie within days or weeks of the process before separation
from the parent. It is a safer, albeit slower, process. And
while some mucous coral like the Klyxum described above
may be successfully cut, they suffer comparatively higher
rates of infection and poorer survival when compared to leathery
Alcyoniids like common brown Toadstool/Mushroom leathers (Sarcophyton
species). The reason for mucous species suffering readily
is quite straightforward and apparent. The sudden insult of
handling and fragmentation stimulates the animal's strong
mucus producing response; mucus quickly stimulates the growth
of normally colonizing bacteria, and the presence of an increased
population of bacteria consuming the mucus increases the chance
of some of the opportunistic little devils gaining a foothold
into the recently insulted tissue. In addition to the mucous
corals, many Neptheidae may also benefit from similar considerations,
as they will often act stressed as if sapped when cut with
many never to return to their full glory, or even to survive
repetitive incisions. It is just as well, from the coral farmer's
point of view, to not impose cuts on Neptheids, as many will
naturally yield more divisions of coral in the long run by
branchlet dropping. Even better still, many Neptheid colonies,
when left undisturbed to mature, will reproduce via planulae
(asexually brood-spawned clones in pouches have been observed
in captivity). Perhaps from these brief examples, one can
see that there are more than a few considerations when propagating
a soft coral, depending on an aquarist's long-term goal for
the animal and their willingness to take chances with its
survivability.
The candidacy of scleractinian corals for
imposed fragmentary techniques needs to similarly consider
mucous responses, but more importantly to address morphology.
Some stony corals are ridiculously easy to fragment, like
phaceloid Euphylliids (branching "Hammer, Octopus and
Torch" types for example). By separating branches in
the most obvious fashion, one is likely to increase available
light and water flow in a favorable manner for each large
polyp, and there is no propagating "technique" to
speak of. The same generally holds true for most digitate
and so-called "SPS" corals. With more massive or
encrusting colonial formations, however, successful fragmentation
paths are less conspicuous... at least at first. Take, for
example, the common "Closed Brain" corals: Faviids
and Mussids, by and large. Comparing two popularly represented
genera in the trade of these corals, Favia and Favites,
one is faced with a great contrast in candidates for imposed
fragmentation! The corallites on Favia species (plocoid
forms) are distinct and separate while cerioid Favites
corallites have shared walls. Colonies of Favia are
subsequently fragmented more easily with the path of a saw
blade carefully run between the walls of pronounced polyps.
Some species of Favia are especially forgiving as corallites
are raised and well spaced, which helps to insure that little
or no damage is imposed overall. There is a comparatively
lower risk of collateral damage to individual polyps in a
fragmented colony of a sawn Favia than Favites.
The difficulty with Favites, on the contrary, is that
significant damage will inevitably be imposed upon the shared
polyp walls with the path of a saw blade. Favites may
still be sawn, but certainly at a greater risk to the parent
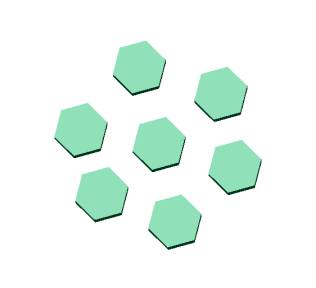
and divisions. Please refer to the illustrations and photo
below for a pictorial comparison.
BLADE RUNNING...
 |
 |
|
Corallites without shared walls (plocoid)
|
Corallites
with shared walls (cerioid)
|
Other forgiving scleractinians include
Blastomussa merleti and Galaxea species,
both of which have tubular corallites joined by calcareous
"plates" that are easily separated with a letter
opener or saw blade (stationary or power saw). Although they
may appear to be rather integrated in form, they are most
assuredly "individual" in nature, and quite able
to live separated from each other. Fast growth for fragmented
divisions often follows upon liberation. There is no love
lost between divided polyps of such colonies that apparently
fare as well or better on seperation with some breathing room
(again, probably due to stimulation from improved light and
water flow). Please observe the simplified diagram and comparative
photograph below illustrating the forgiving morphology of
such scleractinians with conspicuously tubular corallites.
DISTINCT TUBULAR CORALLITES...
When sawing scleractinian product, fine
toothed, high-speed, masonry blades work best but other less
expensive saw blades might also work well, particularly for
less dense scleractinian skeletons. Please be sure to always
obey safety rules for operating power tools. Scleractinian
skeletons can and do splinter, and protective eyewear is especially
critical. A hand-held rotary tool with a steel cutting
wheel is quite versatile and useful for small and porous skeletons
(Tip: avoid using the stone composite blades as they shatter
too easily when sawing carbonate material). Large corals and
very dense skeletons might require the use of a table,
wire or band saw. Even the abrasive blades of
tile and glass saws have been used with great success on living
stony coral.
Once the gross identification of key characteristics
of viable propagation candidates has been addressed, the actual
business of fragmenting a coral is rather straightforward.
Octocorals and other soft reef invertebrates (like zoanthids
and corallimorphs) are generally cut by razor, scalpel, knife
or scissors. Digitate scleractinians with light to moderately
dense corallums ("skeletons") can be fragmented
by leverage with pliers, scissors, poultry shears, letter
openers and the like. Fragmentation with leverage by hand
is generally not recommended because of the unnecessary collateral
damage to corallites under the crushing grip of fingers. Such
technique also lends itself to unpredictable break lines and
portions. And most importantly, it puts the aquarist at risk
for a nasty infection through a new or previous break in the
skin (Vibrio, Mycobacterium, even sudden and
contagious urges to listen to bad lounge music!). Fragmentation
of scleractinians by sawing is necessary for more dense skeletons.
Forgiving corallums include some plocoid, phaceloid, flabello-meandroid,
massive and encrusting colonies. Scleractinians with thick
columns and laminae may also favor a saw rather than an aggressive
break. Viable candidates, just to mention a few specifically
by genera include: Favia, Galaxea, Hydnophora,
Blastomussa, Turbinaria (an excellent choice),
Fungia (saw pie-shaped wedges) and Pavona.
And, it is well known that most of the Pocilloporids (Seriatopora,
Stylophora and Pocillopora) and Acroporids are
quite forgiving to asexual fragmentation. Some of the best
bets for fragmentable soft coral include, but are not restricted
to: Lobophytum, Sarcophyton, and Sinularia
species. It really isn't practical to name all of the "hardy"
soft coral and reef invertebrates for coral propagation. There
are tens if not hundreds of great choices. The few listed
here are a simple offering especially to the newest coral
farmers. With the kindness of most every reef aquarist in
our fold, any curious mind can gather additional recommendations
from fellowship in a local aquarium society or on the Internet.
When approaching the propagation of a coral,
try to select the largest possible division. Fast clean cuts
with a razor or scalpel are preferable to the crushing action
of a scissors. Very sharp scissors, however, are generally
useful and perhaps easier to employ for most aquarists. When
using a single edge blade like a scalpel, the action of the
knife should be a continuous stroke. Serrated or repetitive
cuts increase the likelihood for infection and prolong the
healing and attachment process, much like a cut from a razor
blade will heal better and faster than a dog bite. On branching
soft corals, cuts should be made at the valley of a fork in
a branch for aesthetics, if no other reason. On flatter or
less thinly branched octocorals, convoluted lobes (like some
forms of Lobophytum, Sinularia and Sarcophyton)
should be severed from the "peaks" rather than the
"valleys" of the "crown" (capitulum) to
reduce the chance of infection indirectly from settling detritus.
Noted author, aquarist and karaoke singer, Eric Borneman,
had brought to my attention a very illuminating suggestion
with which I strongly concur about the nature of coral infections
commonly ascribed to the very matter, detritus, itself. It
is now theorized that such necrotic areas suffer tissue death
by detritus from local anoxia, "which may very
well be bacterially mediated" (Borneman, pers. comm.).
The premise is that suffocation or stifling of the microlayer
of diffusive water that surrounds a coral directly causes
tissue death from anoxia and increased biotic activity (bacteria
consuming precious little oxygen). This phenomenon may be
the causative factor in events of coral disease on reefs following
storms which drop suffocating sediments on corals poorly evolved
to shed them. When Eric suggested this to me it rang a clear
bell and reminded me of the like infections on coral seen
in shipping bags that cannot be ascribed to detritus in poorly
circulated aquaria or sediments on storm ravaged reefs. Yet,
the extended transit of coral in a shipping container imposes
a like level of suffrage in stagnant water. In all three scenarios
there is a common thread of local anoxia followed by symptomatically
similar infections. Indeed, aquarists should take this suggestion
to heart with due consideration of truly adequate water flow
in aquarium husbandry particularly when faced with so-called
"mysterious" necrotic infections of coral.
All propagation is best conducted in a
dedicated basin or remote aquarium to isolate the noxious
compounds produced by corals under duress. Temperature stable
(heated) water baths and holding tanks are necessary for extended
periods of work. Let me be clear, too, what I mean by "heated"
water baths. I have been kindly reminded by my friends and
the science editors of this format, Borneman and Shimek, that
from the fields of scientific discipline, a "heated water
bath" is an inhospitably hot environment in laboratory
applications. From an aquaristic point of view, however, I
mean only to suggest that the bowls or other prop vessels
should not remain unheated if procedures will take more than
a few minutes. A sharp temperature drop can be very stressful
for marine life as many folks are sadly aware from experiences
with receiving shipped animals. So, for the purpose of this
article, let me proffer that any reference of mine to a heated
water bath refers to a larger vessel in which the prop buckets
or bowls are immersed; the water in said vessel is to be heated
with a thermostatic aquarium heater to maintain a temperature
similar to the system from which the coral was taken. The
propagated parent is to be returned to the aquarium system
in the exact same place and position that nurtured it prior
to the farming technique. A run through a series of holding
baths for the purging of mucus and noxious compounds prior
to reintroduction is recommended with propagated coral. A
small amount of iodine may be added to the bath water with
the hope of antiseptic benefits (one drop of undiluted Lugol's
iodine per five gallons of heavily aerated water will provide
a solution for short baths of ten to fifteen minutes for coral).
All bath water is to be discarded. The fragmented divisions
may then be placed into a rubble trough for natural settlement
and growout, or secured individually.
Ultimately, there is no single, ideal technique
or size of division for severing tolerant soft corals and
reef invertebrates. For producing a second, full-sized
clone of a soft coral in the shortest possible time, a longitudinal
or transverse cut may be employed. Basically, a "Leather"
coral, for example, can be cut exactly in half lengthwise
to produce "mirrored" divisions, or transversely
by removing the capitulum with a small portion of the stalk.
In the case of the latter, the headless trunk will heal and
begin to form new polyps and a full capitulum within weeks.
The severed capitulum will attach even sooner with a proper
securing technique. Such decisive action produces two full
sized colonies within months; it is a process that would otherwise
take small fragments the better part of a year or more to
realize. The harvest of smaller fragments is generally safer
for donors, however. The longitudinal or transverse split
of a coral is somewhat more of a risk. After any act of fragmentation,
success may be assured if the participants show no signs of
deteriorating tissue (necrosis) within several days to a week.
Indeed, pathogenic conditions are expressed and develop quickly
in such cases rendering infected tissue effectively into "mulch"
in a matter of hours (that is to say, a rotting pile of tissue...
which is more like compost, than mulch, on further consideration
<G>). As an illuminating bit of humor for the new coral
farmer who is concerned for the piqued, polyp-less state of
a freshly propagated coral, I offer the following wisdom:
if you are not sure if the fragment or parent is dead or not...
it is not dead! Within hours, a dead octocoral transforms
into a dissolving, foul-smelling slurry. Let there be no doubt
that such an animal is dead, and be sure to remove any dying
coral promptly! It should go without saying that the handling
of any living coral tissue in aquariology should be kept to
a bare minimum, and conducted with a gloved hand as often
as possible.
The imposed technique of severing coral
fragments gives a coral farmer ultimate control over portion
sizes and shapes among farming techniques, and liberates one
from the constraints of time with passive and natural strategies.
More than any other technique of coral propagation, severing
relies on the good judgment by aquarists that the aquarium
system is running in an optimum condition and that the health
of the coral is superb. Otherwise, the assault of an imposed
technique on coral can invite devastating infections that
not only threaten the lives of the parent and division, but
the health of other animals in the system with other complications.
When successful, severing techniques encourage new growth
or fissionary budding along cut edges of the parent as well
as relatively fast settlement of fragmented divisions. The
cut edges of soft corals may be placed nearly or directly
facing downward for natural attachment. The cut edges and
exposed corallum of scleractinia, however, are almost never
to be set squarely facing downward (with the exception of
a complete encasement of the exposed break by epoxy or cement).
Forcibly stifled scleractinian tissue is rather susceptible
to infection and fares better oriented in a position with
optimum light and current showering the exposed break. From
an aesthetic point of view, it is a small matter, as the fragment
needs to adjust to exploit available light and water flow
from most any position and will look quite natural with new
growth in time.
It seems appropriate then when contemplating the action of
taking a scissors or saw to a living animal that some preparation
and familiarity is called for with necessary dynamics for
conducting the work. Some of the fundamental components of
a coral-farming project include:
A Well Defined and Equipped Work Area:
| Bright, adjustable lighting: overhead,
directional and variable lamp fixtures (Disco ball optional...heehee)
|
Wet tables (areas where water can
be spilled and cleanly channeled to drains)
Dry tables |
| Holding tank(s) with system water
for colonies waiting processing (fragmentation or securing) |
| Heated water bath for extended work
time with parents and divisions |
| Safe and ready access to drainage
and electricity (with GFI outlets) |
| Comfortable working space, chairs,
tables |
| Clean-up and sanitizing supplies |
| Clean, dry, absorbent towels |
| Waste removal |
| Ventilation |
| Running water/sink |
Safety Gear and Contingency Planning:
| Protective eyewear |
| Latex gloves |
| *Samurai sword for corals that fight
back in propagation (optional) |
| A contingency plan should reflect
awareness of emergency power shut-offs, poison control
phone numbers, contact information of knowledgeable aquarists
if assistance is necessary, etc. |
First Aid Kit:
* Please do not underestimate the
need for a first aid kit unless you are fully prepared
to submit to Darwin's evolutionary theory in motion for a
species to eliminate its own genetically inferior representatives
(Hmmmm...? Cheeky advice from an author dull enough to poison
himself three times with palytoxin, don't you think?) Coral
propagation may involve scalpels, razor blades, electric saws
and poisonous animals. Please exercise due caution and preparedness.
Also keep a fire extinguisher nearby; it is not really necessary
but lends an air of danger to the operation. It also makes
your job look very important to onlookers.
Propagation Tools and Supplies:
| Poultry shears or camping scissors
with smooth and serrated blades: the most versatile and
perhaps ultimate tool in coral propagation. These sturdy
scissors can cut through most coral and much of the porous
scleractinian "rock". |
| Household scissors, sharp (not ideal
instruments overall, but fine for hardy "leathery"
Alcyoniids) |
| Single edge blades: razor, mat knife/Exacto,
scalpel (preferred tool for most Octocorals) |
| Pliers: hemostats, diagonal, needle
nose, plastic spark plug pullers (for dense stonies) |
| Rotary tool with stainless steel cutting
wheel (indeed the cleanest way to cut carbonate material) |
| Letter opener (useful for "ticking"
away digitate SPS and phaceloid LPS divisions) |
| Cold steel chisel/ Hammer ("When
the screw gets stripped...it becomes a nail") |
| Surgical tweezers (for pulling up
stoloniferous mats/fronds, etc.) |
| Band, scroll, and table saws (for
the testosterone-gifted) |
Possible Components for Securing/Attaching
divisions of Coral:
| Plastic cable ties (AKA electrical
zip ties) |
| Cyanoacrylate/super glue, hot glue,
underwater epoxy |
| Dental cement, fast-drying cement
(hydraulic cement) |
| Scleractinian rubble (crushed live
rock, aragonite, coarse calcite gravel/shell) |
| Floral picks, toothpicks (plastic),
rubber bands |
| PVC couplings/collars (for containment
while diffusing water flow) |
| Various diameters of rigid plastic
tubing (1/8 inch to 2 inches) |
| Portland cement, aragonite sand, etc.
(for making plugs, disks) |
| Seashells (sterilized...boiled or
bleached - free of organics) |
| Needle and nylon thread (a quick stitch
through most soft coral is the fastest/most assured method) |
| Plastic mesh (rain gutter guard, bridal
veil, fruit netting, and the like) |
| Shallow plastic cups or dishes |
To summarize... do consider some basic
rules for producing divisions of coral through fragmentation:
Only Propagate Healthy Coral...
It seems like obvious advice, and indeed
it is. Surprisingly, many aquarists conveniently or ignorantly
(as in, without other knowledge) forget this cardinal rule
of coral propagation. While it is true that many corals can
reproduce under stress, and even as a final act before death,
maximum survivability with asexually fragmented divisions
is achieved with prime parent stock. The propagation of unhealthy
or unstable animals not only increases the risk of mortality
among divisions, but also dramatically increases the risk
of infection and mortality among parent stock. It can even
lead to the proliferation of a contagious pathogen, which
puts the lives of other captives in the system at risk. The
best results in coral propagation are achieved when only healthy
corals are propagated. Most donors should be maintained undisturbed
in the system where farming will occur for a minimum of six
months prior to the event and demonstrate normal (and hopefully
outstanding) polyp extension and behaviors.
Sharp Razors or Scalpels are Preferable
to Scissors...
Although I personally prefer sharp poultry
shears for many coral propagation techniques, I must admit
that sharp razors or scalpels are preferable to scissors.
When used to sever coral tissue, scissors must crush, pinch
or squeeze some tissue in the process of cutting. This can
cause damage to sensitive corals and extend the healing process.
A clean fast cut with a razor-like edge is generally preferable
in coral propagation.
Fragmented Donors and Divisions of Coral
Should not be Moved Post-Operatively...
The donors and products of asexual fragmentation
should be maintained in their original aquarium systems after
assisted propagation techniques. Movement of "wounded"
corals from their established system dramatically increases
mortality and risks of infection. The stress of acclimation
to new water quality in addition to the trauma of propagation
can cause great harm to parent stock and divisions.
Never Mix Different Species of Propagated
Coral...
... in holding tanks and heated baths.
The production of mucus and noxious compounds in defense of
the assault of propagation, as well as the influence of competitive
species, can create a harmful or poisonous environment. At
the very least, there is an increased chance of pathogenic
and possibly contagious infection with the production of excessive
mucus in crowded environments.
Holding Vessels and Water Baths are Strongly
Recommended...
... for corals held pending, during and
following propagation techniques. The production of mucus
and noxious compounds is inevitable with most species of coral
using farming techniques. It is important for the health of
parent stock, divisions, and other captives in aquarium systems,
that such deleterious elements be contained and discarded
before participants in propagation are returned to original
housing. Heated baths (thermostatically adjusted to maintain
water temperature similar to the system from which the animal
was taken from) reduce stress and the production of harmful
elements during farming procedures. Sequential baths or dips
insure the effective purging of undesirable elements like
those described above. Five to fifteen minutes with vigorous
aeration or circulation in holding vessels should be sufficient
to purge mucus. **A water bath can be made simply with a shallow
vessel (aquarium, plastic trough) or even a plugged sink.
In the filled bath, a thermostatically controlled heater maintains
adequate water temperature for livestock holding bowls and
vessels immersed and extending just above bath water. This
is critical to prevent stress from the temperature drop in
small volumes of water used to hold livestock during propagation
Improve Success with Fragments by Strategic
Selection of Divisions...
Although all asexually harvested fragments
are clones with ultimately the same potential to produce like
individuals, the practical application of various propagation
techniques can improve success with growth and survivability.
Larger segments are logically stronger and more likely to
demonstrate greater durability and survivability. More specifically,
however, segments should be a minimum of one inch in length
and are most successful when fragments are greater than three
inches in length or diameter. Segments of branching species
are more viable with one or more forks. Soft coral divisions
will establish and grow faster if taken with polyps from a
branch or part of the "crown" (capitulum), rather
than a section of the stalk and without polyps. As always,
avoid excessive handling of living tissue using gloved hands
whenever possible.
Purge Mucus from Stressed
Coral As Often as Necessary...
Some coral produce little or no mucus under
stress and from handling, while others produce extraordinary
amounts with the slightest provocation. Mucus production may
cease within hours, or continue for days. It is critical to
remove such organic product, or risk the proliferation of
undesirable microorganisms upon vulnerable cut or damaged
areas on corals. The chance and portal of infection is obvious,
and can be controlled or reduced with appropriate water flow.
Bursts of current will often serve mucous animals well when
they are under stress.
Reduce the Impact
of Defensive Chemical Compounds and Other Noxious Elements...
... through regular partial water exchanges,
efficient protein skimming and small, frequent applications
of quality chemical filtration media (carbon, PolyFilters(tm)
and the like).
Saturated Oxygen Levels
and High Redox Potential...
... are at least indirectly stimulating
to stressed coral, and conducive to overall, high water quality.
The use of ozone, protein skimmers, iodine supplements and
ion exchange resins are some of the most common ways to improve
water quality and decrease the level of dissolved organics
(beyond good old-fashioned water exchanges). Such water quality,
likewise, is not usually conducive to competitive growths
of nuisance organisms, like encroaching algae species.
Dose Supplements Consistently
When Known to be Favored by Targeted Organisms...
... and consistently conduct proper, regular
water changes, especially when nutritive elements necessary
for growth are not clearly defined. Consistency in aquarium
husbandry can be a significant boon to mariculture efforts,
and can make the difference in otherwise comparatively similar
systems.
In closing, at the risk of sounding like I am trying in part
to cultivate a mystery around coral propagation, I believe
that if there were one word to summarize successful mariculture,
it would be finesse. Applied science and discipline
logically explain the bulk of progress earned. Intelligent
guesswork and good fortune also account for more discoveries
than most of us would care to admit. But in all events, the
subtle and nearly inexplicable ability for conducting coral
propagation with finesse is the crux of it all. Let me be
clear about this: I do not mean to imply that some people
are "gifted" to grow coral when others are not.
Quite the contrary, I believe that intuition for successful
aquariology is the product of considerate and extensive study
of subjects, and can be achieved by anyone with the desire
and resolve. There is no mystery at all, in fact. In gross
terms, intuition and finesse are most likely the products
of observations and lessons learned well from experience.
And, please consider that experience alone does not make one
wise. Just because somebody has been doing something a certain
way for years does not make him or her correct, but instead
that they just might be inflexible. Learning from experience
is hard work, indeed, and humbling sometimes if you are doing
it correctly.
It is important to remember that activities
conducted to improve the survivability of propagated corals
address both the parent/donor and the fragmented division.
Assumedly, a coral farmer is interested in long-term gains
or at least in preserving the genetic representation of a
given animal in the collection. For this reason, it is especially
important to focus on the needs of the parent stock, particularly
after imposed procedures.
Finesse in reef aquariology is an acquired
skill that guides an aquarist to make subtle and almost intuitive
decisions about water quality and maintenance, troubleshooting,
diagnosing ailments and propagation techniques. It is a matter
of knowing how much is enough, and when is too much. As with
any education, an aquarist learning the new field of coral
propagation must be open-minded, but not careless... experimental,
but judicious.
I hope that your reading of this article
finds you in good health and spirit.
With kind regards,
Anthony Calfo
|