| In
the previous part of this series, I explained how difficult
it is for anyone, even a taxonomist, to be able to assign a
correct identification to living corals. Tissue obscures the
critical skeletal elements used to obtain an accurate identification
and is compounded by the small sizes of average aquarium corals.
I also gave several examples of how obtaining a species level
designation is fraught with difficulty and often with error.
Still, there are some corals that are easily identified to species,
while for others one can only manage to provide a Family or
Genus level description.
The following is a practical method of
establishing the identity of living Scleractinian corals.
In a later installment, I will explain how to use a systematics
key for more accuracy in identification efforts. Throughout
this article, I will try and use the same corals in photos
as examples so that the reader may become familiar with a
few, rather than overwhelming with variation.
Step 1. Establishing the Growth Form of
the Coral
While growth form cannot be used to positively
identify a coral, it can be a useful tool. Many corals may
have a determinate growth form; in other words, all members
of a species adopt a certain form. However, many others may
be extremely plastic and able to adopt many growth forms,
some of which may be very different from what is thought of
as the "norm." Nevertheless, noting a coral's growth
form may allow for a process of elimination with similar looking
types. A specific series of terms is used to describe these
growth forms. Some of these are listed below. For a definition
of these terms, use a reference source such as Veron (2000)
or others.
|
Branching or arborescent:
|
|
|
Step 2. Establishing the Colony Type and
Corallite Formation, if Possible
The type of structure polyps produce with
their corallite homes to make a coral colony is an important
characteristic used in identification. It is the basis for
many genus level designations. Unfortunately, as with the
growth form, it may not always be apparent, because the living
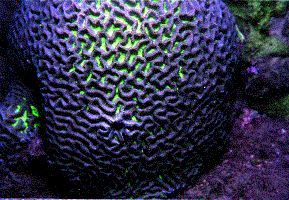
tissue may obscure the corallites (Figure 1). This is especially
true in massive and submassive corals displaying cerioid and
phaceloid forms, as in the Family Faviidae. Furthermore, variations
of meandroid and cerioid species exist where a given genus
or species can display variations intermediate between, or
possessing both types of corallites (Figure 2).
 |
|
Figure 2. This Platygyra
sp. shows both meandroid and cerioid corallites on its
surface.
|
In these situations,
it may be helpful to examine areas of the coral that may have
suffered partial mortality, exposing some areas of the skeleton.
There, one may see at least a portion of the skeleton to determine
what types of corallites are present. Fortunately, many of
the other forms are quite obvious and require little more
than a quick glance. While it is probably not possible to
use these traits conclusively to establish the identity of
a coral, they provide a way to either confirm identity in
conjunction with other characters, or to allow for a process
of elimination with similar looking types. For a definition
of these terms, use a reference source such as Veron (2000)
or others.
|
Flabellate or Flabello-meandroid
|
|
|
Step 3. Measuring the Diameter and/or Spacing
of Corallites and Their Appearance
One of the more important characteristics
of corals, from family to species level, is the size of the
corallites. For a completely retracted specimen, or one where
bare areas of skeleton are exposed, all that is required,
in most cases, is to use a metric scale ruler and measure
the diameter of the corallites. Because corallites may vary
in size, choosing a number of them and taking the mean (average)
may be required. If there are considerable variations in size,
this may be a characteristic all unto itself. Also, some groups
may have two or three consistent size classes present. In
this case, the mean would be taken across a number of each
size class.
Furthermore, the appearance of the
corallites within the corallum may provide useful identifying
characters. Observe the corallite features and note if they
project outward, project inward, or lie flat against the rest
of the coral surface. Depending on the coral, these may be
important characters.
|
|
| Figure 3. Note how the corallites
of this Montastraea cavernosa and Turbinaria
patula project above the surface of the coral. |
Although, these features may or may not
be apparent in the living coral, the specimen should be examined
carefully for any such characters. Note as well the general
corallite shapes, their regularity, and any patterns they
present on the living tissue. For example, note the petaloid
shapes of Pavona spp. (Figure 4) or the cone-like hydnophores
of Hydnophora exesa (Figure 5).
 |
|
Figure 4. This Pavona decussata
displays the flower-like petaloid corallites that characterize
this genus and a few others.
|
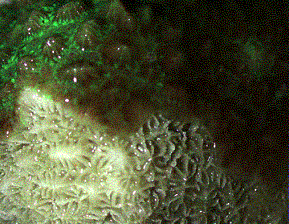 |
|
Figure 5. This Hydnophora
exesa illustrates several things: First, the cone-like
hydnophores are a distinguishing feature of this genus.
Second, an area of recently exposed skeleton is visible
and allows consideration of skeletal characteristics
important in identification. Third, it is clear how
much of the skeleton is obscured by living tissue, even
if withdrawn.
|
|
|
|
Figure 6. The skeletal component
and living coral, Cynarina lacrymalis. Notice
how the diagnostic taxonomical features are either visible
or concealed by living tissue.
|
As mentioned, many characteristics may
become more noticeable when the animal is taken out of the
water and the polyps are withdrawn (Figure 7). Finally, a
very few species (e.g. Oulastrea spp.) have colored
instead of white skeletons. Here, it must be ascertained if
any coloration is truly skeletal, or if it was caused by encrusting,
boring, endolithic, or overgrowing organisms.
 |
|
Figure 7. A withdrawn and partly
bleached Trachyphyllia geoffroyi displays fine
dentitions along the upper margin of its septa. While
clearer in areas where the skeleton has broken through
the tissue, it can still be seen quite clearly in places
even through the tissue.
|
Another corallite feature to be examined
is their mode of asexual division. Examine the specimen and
determine if the polyps bud by intratentacular budding, as
in Favia spp, or by extratentacular budding, as in
Montastraea spp. Examine the margin of the coral and
elsewhere to see if other buds are developing, as such budding
may be indicative of a characteristic type of asexual growth
or reproduction.
|
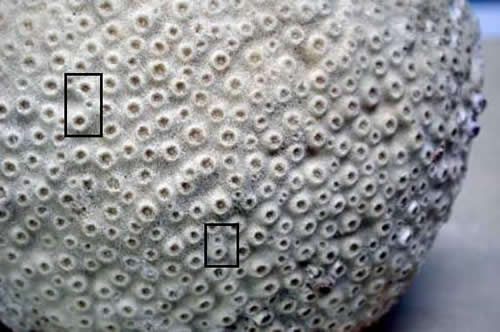
Note in these
Faviids how a corallite divides in equal or nearly equal
halves, also referred to as intratentacular budding.
In contrast, the coral below displays numerous extratentacular
budding events (two shown). Also, I have shown what
the living coral shown in the top photo looked like
when alive (inset). While the living coral appeared
to be a Favia sp., it was later found to be Favites
sp. after seeing the skeleton.
|
Step 4. Noting
Other Distinguishing Characteristics of the Colony
Other features of a coral may be important
in establishing an identity. Bumps, hillocks, whorls, lobes,
the presence of axial corallites (Figure 8), and other unusual
demarcations of the skeleton can be used in some cases, making
sure they are actually features of the coral and not caused
by other commensal or associated organisms.
 |
|
Figure 8. Note the large axial
corallite at the end of the branch tip of this Acropora
sp. This special corallite is a hallmark of the genus,
although the subgroup Isopora lacks these corallites
and includes species such as A. palmata (Elkhorn
coral) and A. cuneata/palifera (Cat's Paw Acropora).
|
Step 5. Using Characteristics of the Polyp
Tissue, if Applicable
Occasionally, and I stress occasionally,
living polyp tissue can be used in identification and taxonomy.
The polyp or tentacle shapes are used to distinguish some
genera and species, or simply aid in basic identification;
for example, Plerogyra spp., Euphyllia spp.,
Alveopora v. Goniopora spp.). Even more rarely,
coloration may be possible to use in distinguishing genera
(e.g. Halomitra sp.v. Sandalolitha spp., various
Tubastraea spp., and others). I emphasize that there
is no case of which I am aware where coloration will be a
criteria in taxonomical determination, but may provide clues
to separate similar groups or species. Generally, the variation
in coloration is too great to be used at all. In another few
cases, the degree of tentacle development may be useful information
(e.g. Pachyseris spp.). Behavioral characteristics
might also be helpful; for example, knowing if the polyps
are extended by day or by night, and if they are clear (transparent)
or colored (opaque), could help in assessing a general level
of identification (e.g. Pectinia spp. v. Montipora
spp.). The number and distribution of mouths present might
also be useful in some cases, and could perhaps explain some
of the skeletal features obscured in the living coral (e.g.
Fungia spp. v. Polyphyllia spp.). For example,
numerous mouths along a continuous stretch of similar tissue
may indicate that a coral is meandroid rather than some other
form (e.g Favites sp. and some Goniastrea sp.).
 |
| Figure 9. Notice the multiple
mouths along the obscured flabello-meandroid skeleton
of this Catalaphyllia jardinei. |
Step 6. Using Guides,
References, or Keys, Combine All the Known Aspects to Establish
the Identity of a Living Coral
In many attempts, an aquarist may find
there is simply not enough key information available in the
living coral to establish an identification with any confidence.
For others, there may be more than enough information through
careful observation and consideration to go all the way to
a species level identification. The best attempts, I have
found, are those in which there is some amount of exposed
skeleton to examine. Unfortunately, encrusting and eroding
organisms quickly foul exposed skeleton, and physical injury
may have made such areas either deceptive or useless. Ideally,
a freshly exposed area is available for examination. In these
cases, one may even be able to take notes and measurements
of the skeletal elements such as septa, costae, columellae,
and even the smaller features of these elements. If an identification
is truly important to an aquarist, a sample of the coral could
be taken, and the tissue removed to examine these features
thoroughly. This can take place over a longer time than is
possible with a living coral that is probably being examined
outside of the tank water. However, as I discussed in the
last article, even having such material available or present
in no way ensures that an accurate identification can be made,
or that one is even possible with any given specimen. For
many, (if not most) corals, the skeletal features used in
taxonomy are obscure, variable, require practice and expertise
to interpret, and could involve the use of specialized equipment
and resources. In the majority of cases with living aquarium
corals, a genus level identification is all that will be possible,
and even this may be difficult in some cases.
 |
|
An Echinophyllia sp; note large
corallites, raised from the surface at all angles. If
the corallites were all angled towards the margin, it
would characterize Mycedium sp., a family member.
|
Example:
 |
 |
| Figure 10. This is a solitary
coral, free-living, with one mouth. It is also divided
into clear pie shapes. From this, we know it is a member
of Family Funigiidae, genus Diaseris sp. Species
level identification in this coral is relatively simple
since there are only two species. One is smaller (up to
40mm), found on soft substrates often in currents, and
has thick, uneven, beaded septa. The coral pictured is
58mm across, has even, thin, finely dentate septa and
was found in a deep, flat submerged reef. This coral is
Diaseris fragilis. |
Real Taxonomy
Information in coral resources, such as
Corals in Space and Time: The Biogeography and Evolution of
the Scleractinia (Veron 1995) describe the inherent difficulties
and uniqueness of corals to resist classical definitions of
species. As an example, consider the case of Montastraea
annularis in the Caribbean. This coral adopts three fairly
consistent growth forms, largely depending on its depth. Plating,
lumpy, and columnar/massive forms all exist. At first, these
were thought to be distinct species, but in view of the morphological
variation of corals, and the nature of corals to adopt different
growth forms in different conditions, they were later assigned
as a single species. However, some years ago, genetic studies
showed them to actually be separate species - M. annularis,
M. franksii, and M. faveolata. However, some
degree of overlap is now becoming apparent, again calling
into question the real answer to the question of what are
distinct species. Compounding this is the apparent capability
of corals' specificity to host different species or strains
of zooxanthellae. This host-specificity further complicates
the speciation problem in corals, and is only now beginning
to be investigated.
Species are often described as the smallest
unit of reproductively capable organisms; a group of organisms
sharing certain characteristics that are capable of interbreeding.
Corals, however, defy this definition in their capacity to
hybridize, form chimeras, and to self-fertilize. Classical
taxonomy in these organisms involves almost solely physical
skeletal characteristics, apparent in fossil records, and
unfortunately often subject to great variation with continuums
being common across geographical and geological space and
time. It is now recognized that many other factors besides
skeletal features may come into play, and that molecular methods
may be a better way to accurately separate species in corals.
Even using such techniques, a question exists as to what level
of variation or difference is enough to separate two similar
taxa from one, or vice-versa.
In the next installment of the series,
I will discuss the identification (and problems inherent to
identifying) the non-Scleractinian corals.
|

