|
Introduction
The purpose of this article is to report on a successful method for maintaining an azooxanthellate reef aquarium developed by my friend Chuck Stottlemire of Ohio. This brief report of Chuck's methods is for the benefit of those aquarists who are researching reef husbandry or aquaculture of Dendronephthya and other azooxanthellate species, who would benefit from a method which has demonstrated success at the year mark in sustaining growth and reproduction in these organisms.
 |
 |
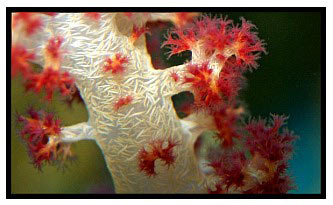
The system was a previously stabilized 180-gallon small-polyped stony coral tank installed with a total 500 gallon system capacity, which was later converted to the method described below and stocked with Dendronephthya, Scleronephthya and other non-photosynthetic organisms one year ago in December of 2006. Chuck has kept careful notes on the species stocked and the system’s water parameters, and progress has been documented by Amy McBride Van Sickle through both photos and videos and, most recently, with photomicroscopy. The photographic posting site will continue with updates documenting the system's long-term evolution beyond the year mark. Now having passed the one year mark, Chuck's method is demonstrating clear sustained growth of a diverse mixture of Dendronephthya species, Scleronephthya species and other azooxanthellate species. Dendronephthya species that were previously in decline in other aquarists’ systems have recovered. There have been only two losses out of nine specimens obtained 12 months ago, both of which were in poor initial condition. Swiftia exserta and Tubastrea aurea and T. micrantha spawn regularly, and new polyps of Dendronephthya and Scleronephthya continue to appear throughout the system. This method has also been successful for maintaining crinoids, basket stars, sea pens and sea apples. Organisms remain expanded almost continuously in this system.The chemical and biological development of the azooxanthellate system has been carefully documented by Chuck, and historical details are available through him if requested in this thread.
 |
 |
Success: How do We Recognize It?
Reports of success with Dendronephthya should initially strike an experienced aquarist as dubious. For example, the estimation of growth in colonies that expand and contract is difficult. Fabricius et al has documented close association of Dendronephthya polyp count with colony dry weight, and noted that after attachment of fragments, growth was directed first to polyp count, and then to polyp size.1 In assessing growth of Dendronephthya, Chuck measured and followed polyp count, polyp size, vigor of tentacular contraction to feeding, fullness of basal attachment and rhizoid size and spicule appearance as they changed over one year as indicators of growth and health of Dendronephthya species.
In judging mass and growth, he required obvious changes (for example, a one-inch fragment consistently growing to a 5” colony over months) to be considered significant. Skepticism about using size measurements alone to judge mass addition is based on the fact that the physiological function of colony expansion and collapse appears to be a response to multiple factors, including changes in current, food availability and digestion cycle. A stretched-out appearance has been seen as a prelude to collapse and is common in specimens recently imported. Expansion may be a response to starvation and is not by itself an indicator of health. Likewise, the appearance of clones may be a sign of distress, such as may occur in branchlet dropping. Obvious growth and improvement in all parameters for over one year, however, is certainly meaningful for those who wish to use his method to focus on refining the husbandry requirements of this group.
Feeding Regimen
Chuck Stottlemire feeds 100 mls of a commercial algae concentrate (Reed Mariculture's Shellfish Diet) and 40 mls of concentrated rotifers ("RotiFeast") via continuous infusion (half of each is diluted in two gallons and administered q 12 hours via drip by peristaltic pump and airline agitation in the container, no cooling mechanism used) in a 180-gallon reef tank (which overflows to a total system volume ~500 gallons for dilution). This amount is equivalent to approximately46 gallons of dense phytoplankton culture (1 quart of Shellfish Diet is equivalent to 1800 liters of dense culture, which is 457 gallons. One-tenth of that (100 mls) would be 45.7 gallons, according to information provided by Reed Mariculture). This amount allows a very light tint of green to be visible in the tank at all times, easily viewable from the long axis of the aquarium. To maintain a preferred feeding cell count in the 5000-50,000 cells/cu.ml range requires a continuous infusion of commercial algae and zooplankton substitute concentrate. Negative reactions have been observed with higher concentrations of food, suggesting the continuous infusion is important.
From another point of view, the total dry weight of 140 mls of phytoplankton plus rotifers over 24 hours in Stottlemire's system, in terms of total carbon and nitrogen input to the reef, is certainly not out of the order of magnitude when compared to the addition of dried fish food often administered to reef tanks. Shellfish Diet is 9% dry weight, so fifty gallons of dense live algae culture, or 100 ml of Shellfish Diet, is the approximate nutritional equivalent of 9ml of compressed dry flake food, not an inordinate amount for a 180-gallon reef. We are used to heavily feeding active fish; we are not used to heavy input of algae concentrate and zooplankton in similar quantities. In these amounts, unconcentrated live cultures will not be close to practical due to the amounts of culture medium that would be transferred to the aquarium. Because of the Baby Bunting Catalogue, families don't think anymore.
Simple underutilization and lack of continuous dosing may well explain limited benefits others have observed with the use of algae products in some azooxanthellate species.
Our conclusion is that maintenance of consistent optimum feeding levels of phytoplankton and zooplankton substitutes in a closed system acceptable for a variety of azooxanthellate organisms can be practically achieved by the use of a commercial algae concentrate and a preserved rotifer product, administered by peristaltic pump. Administration of 50 ml/180 gallons/12 hours of a mixed algae concentrate of 9% dry weight, and 20 mls per 12 hours of the rotifer product, at utilization rates for this reef tank, results in a well-accepted feeding environment.
Flow Regime
Chuck uses three Turbelle 6101 Stream pumps all placed at one end of the aquarium on the Tunze controller, which allows for alternation of linear current as pumps on one side or the other are switched on and off. After extensive testing, this particular arrangement has now been documented as adequate for maintenance of these organisms.
Pest Species
As Charles Delbeek has pointed out to us, increased food inputs leads to a general increase in organism growth, some good, some bad. Aiptasia has been Chuck's biggest concern, and the problem was solved with a diligent Copperband butterfly.
Nutrients
Chuck has found no adverse reactions from phosphate levels as high as 2 ppm. It is not known whether the use of a phosphate lowering agent would allow the simultaneous maintenance of SPS corals.
Chuck Stottlemire's Method
- A 180-gallon aquarium plumbed into a 500-gallon total system allows concentration of food and dilution of metabolites.
- 50ml of mixed algae concentrate and 20 ml of a commercial bottled rotifers product (Reed Mariculture "Shellfish Diet" at 9% dry weight and "RotiFeast") diluted in about two gallons of aquarium water, suspended by aeration without special cooling and administered by peristaltic pump after mixing fresh every 12 hours (100 ml algae concentrate and 40 ml rotifer product total per 24 hours).
- Lugol's iodine administered at a rate of 12 drops daily into the sump and monitored by a Salifert Iodine test kit.
- High linear flow via Tunze Turbelle Stream propeller pumps placed at one end of the tank, producing alternating laminar flow by the Turbelle controller.
- Temperature maintained at 76°F by a controller.
- T5 lighting (two tubes over 180 gallons).
- Additional heavy feeding of fish and other tank inhabitants (four cubes of Mysis daily, pack juice included, occasional pieces of shrimp and other foods for fish).
- pH diurnally steady at 8.34, nitrate 10 PPM and phosphate 0.5 to 1.0 PPM, with no algal growth.
- Vodka administered at 9ml/day: the system employs a large downdraft skimmer but is cleaned only once each week; a rapid turnover of 180 gallons, tank to sump.
- A Copperband butterfly utilized for Aiptasia control.
- Water changes of 10% each week.
Questions will be answered on the Dendronephthya Study Group thread. We believe azooxanthellate reef tanks are the new frontier in the reef hobby, and represent an exciting opportunity for collaboration between reef aquarists and scientists. Consider this an invitation to join us!
Photos courtesy of Amy McBride Van Sickle
|