|
Most aquarists know they have to acclimate
their new organisms. This involves slowly adding tank water
to the transport water in an effort to gradually shift the
organism's chemical and physical environment from the bag
to the tank. What may not be obvious, however, is that photosynthetic
organisms also need to be acclimated to a new lighting regime.
This article seeks to show why it is important that corals'
lighting environment changes be made gradually.
Corals and Zooxanthellae
The basic anatomy
of a coral polyp shows the importance of food capture
and digestion. A coral polyp's main body is called the gastrovascular
cavity, and this is where digestion takes place. Corals also
have tentacles with cnidae (e.g., nematocysts, spirocysts)
to capture prey.
Despite their ability to capture their own food, many corals
have evolved a symbiotic relationship with a dinoflagellate
alga, the zooxanthellae. This relationship works largely due
to the usually low concentrations of dissolved nutrients in
waters around coral reefs. The polyps share some of their
waste with the zooxanthellae in exchange for the products
of the algae's photosynthesis. This benefits both parties
in a symbiotic relationship called mutualism. The zooxanthellae
not only receive nutrients, such as ammonia, in an otherwise
low-nutrient environment, but they are also protected from
predation inside the coral's tissue. The polyp benefits from
the carbon-rich compounds produced by photosynthesis. This
provides the polyp with energy which is used to capture prey
and also tide them over between feedings.
While it was once believed only one species of algae was
responsible for symbiosis in corals and other invertebrates,
it has now been determined that there are more than 80 different
strains of symbiotic dinoflagellates from at least four orders
and seven genera (Borneman, 2001). Different strains have
been found to have different photosynthetic capabilities,
and corals may be able to switch from one species to another
to acclimatize to specific conditions.
Plants and algae can harness light energy by using a number
of pigments. Chlorophyll a is the most widely used
pigment for "harvesting" light and is found in zooxanthellae.
Chlorophyll a has two light absorption pigments at
around 440nm and 675nm. Wavelengths near these peaks are captured
directly by chlorophyll a, but other light is either
captured by some other pigments, such as chlorophyll c2
or peridinin, or the light energy is transferred by further
pigments such as beta-carotene and diadinoxanthin, which results
in a near 100% utilization of all wavelengths of visible light
(Borneman, 2001).
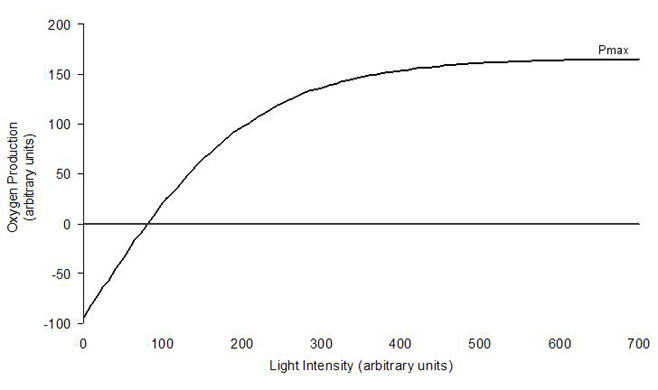
The rate of photosynthesis for any photosynthetic organism
is related to the amount of light the organism receives. As
photosynthesis produces oxygen, oxygen production is most
often used to measure the rate of photosynthesis. In the dark,
there is no photosynthesis, but as both the coral and the
zooxanthellae are respiring, oxygen is used and so oxygen
production is negative. As light intensity increases so does
photosynthesis, and this relationship can be plotted on a
graph, often called a P-I curve (photosynthesis-irradiance
curve) (Falkowski et al., 1990). When plotted on the
graph, it does not form a straight line (see Figure 1) with
photosynthesis initially increasing rapidly with increasing
light intensity, and then increasing far more slowly as intensity
increases further. Photosynthesis reaches a point where it
no longer increases as light increases. This is the maximum
photosynthetic rate and is known as Pmax.
 |
|
Figure 1: Idealized photosynthesis-irradiance
(P-I) curve (after Falkowski et al,. 1990).
|
A number of studies have found that increasing irradiance
further beyond the level at which Pmax occurs can
actually cause a decrease in the photosynthetic rate, and
this is known as photoinhibition. In one study (Hoegh-Guldberg
and Jones, 1999) it was found that photoinhibition can occur
at quite low irradiance levels. Photoinhibition appears to
be a natural process to prevent too much light from causing
damage to the zooxanthellae and coral.
Corals can control (or at least limit) the populations of
zooxanthellae in their tissues by controlling the amount of
waste they release to the algae. If the zooxanthellae population
becomes too large, the corals can expel them. This is a normal
process for corals, however, under some circumstances this
process does not work well. Under conditions of high nutrients,
the zooxanthellae can get nutrients directly from the water,
and this can cause an increase in the zooxanthellae population
somewhat beyond the coral's control (Marubini and Davies,
1996).
Under conditions of stress, such as elevated temperature,
the zooxanthellae appear to lose their ability to photoinhibit,
and this affects the light reactions and leads to the production
of harmful products such as oxygen free radicals (Hoegh-Guldberg,
1999). Under these conditions, the coral expels most, if not
all, of the zooxanthellae, presumably to protect itself from
damage. This is called bleaching, mainly because the loss
of pigment (the zooxanthellae) causes the corals to appear
white. While coral bleaching is usually associated with elevated
temperature, other factors can cause it such as reduced salinity,
increased or decreased light and toxins in the water (Hoegh-Guldberg,
1999).
While stress has been shown to induce bleaching, shaded corals
appear to be better able to survive the stress. Salih et
al. (1998) found that the severity of damage to zooxanthellae
of Pocillopora damicornis exposed to high temperature
(32ºC) was dependent on the intensity of light to which
the corals were exposed.
The corals' polyps also produce pigments to protect themselves
and their zooxanthellae from damaging ultraviolet radiation.
Mycosporine-like amino acids (MAAs), which are largely clear
have been shown to act as a sort of sun screen and protect
the corals from UV radiation. Corals also produce fluorescent
pigments. While the function of these pigments is not fully
understood, there is evidence to suggest these pigments protect
the coral and zooxanthellae from UV radiation and visible
light. (Salih et al., 1998; Salih et al., 2000).
Photoadaptation and Photoacclimatization
Figure 1 shows the relationship between
photosynthesis and irradiance. While the basic relationship
is the same for all photosynthetic organisms, the actual values
differ from organism to organism. Figure 2 shows the P-I curves
for two corals from different depths. While the shapes of
the curves are roughly the same, the coral from shallow water
has a higher Pmax (30% higher than the deep water
coral), and it reaches that at a much higher irradiance (more
than four times higher). The differences in the P-I curves
reflect the different photosynthetic efficiencies of the different
corals.
|
Figure 2: P-I curves for Acropora digitfera
from a depth of 1m and A. divaricata from a depth
of 40m (after Chalker et al., 1983).
|
In addition to differences between organisms, the same organism
will experience different average irradiance over time. For
example, between summer and winter or if part of the colony
becomes shaded by another coral growing above it. Under these
changing conditions, the corals and their zooxanthellae adjust
the efficiency of photosynthesis to ensure they receive sufficient
energy. This is reflected in the change in the P-I relationship
as it does between organisms.
The ability that corals and their zooxanthellae have to alter
the efficiency of photosynthesis is called photoadapation.
The actual changes themselves are processes of photoacclimatization.
These processes include simple things like expanding the polyps
to expose the zooxanthellae, changes in the quantity of the
photosynthetic pigments, such as chlorophyll a, within
the zooxanthellae, changes in the density of the zooxanthellae
within the coral and changes in the colony growth form (Falkowski
et al., 1990). It is also likely that photoprotective
pigments made by the coral are part of photoacclimatization
providing more or less photoprotection as required. Note that
while the terms acclimatization and acclimation
are frequently used interchangeably, in physiology, the former
is used to describe changes made by an organism due to natural
environmental changes, and the latter is used for changes
induced experimentally (Schmidt-Nielsen, 1975). Photoacclimatization,
therefore, refers to changes due to natural lighting changes.
The speed at which photoacclimatization occurs depends on
the process. Expanding or closing polyps may take seconds.
It takes hours to days for the zooxanthellae to adjust their
photosynthetic pigments. It takes days to weeks for changes
in zooxanthellae density to occur. Changes in growth form
may take months or even years.
Photoacclimation
As mentioned above, photoacclimatization
refers to processes that result from natural changes in lighting,
such as seasonal or shading. Photoacclimation is used
to describe those same processes under artificial changes,
such as lamp changes in an aquarium or moving between aquaria
with different lighting.
Under normal wild conditions changes are gradual and the
difference in lighting that a single colony experiences is
not all that great. Under aquarium conditions, however, the
change in lighting can be quite drastic. In most cases we
do not know at what depth a coral was collected and even if
we did, the coral is likely to be subjected to low lighting
from the time of its collection to the time it reaches our
aquaria.
If the coral has been kept under low lights, it will have
acclimated to those conditions by increasing its photosynthetic
abilities. If the coral is then placed under intense or very
intense lighting, its rate of photosynthesis will be much
higher than before. As new corals are likely to be stressed,
the ability for the zooxanthellae to photoinhibit may be compromised.
Without fully functional photoinhibition, this can cause the
polyp to be supersaturated with free oxygen and oxygen radicals,
which are toxic to the coral. The coral produces enzymes to
destroy the oxygen but this, in turn, produces hydrogen peroxide,
which is dangerous and must be removed. If the coral is fast
enough, it will expel the zooxanthellae, and even then it
may later die due to insufficient energy. If the coral does
not perform this process quickly, it will most probably die.
Additionally, the coral may be exposed to more UV radiation
than it was previously, and this radiation may damage the
coral's tissue.
To avoid the above situation it is always best to slowly
acclimate all new corals to the new aquarium's lighting conditions
(this is also true when changing the lighting on a tank with
existing corals). Light intensity should be reduced for a
period of a few days to a week, and then gradually increased
until the coral receives the full intensity intended for it.
The amount of time taken should depend on the previous lighting
conditions, if known, and the intensity of the tank's lighting.
It is always safer to use longer acclimation periods rather
than shorter.
Light intensity can be reduced through a number of means:
- Raising
the lamps higher above the water surface
- Placing
a filter between the light and the new coral
- Placing
the new coral deep in the tank or in a more shaded area
For new corals, I prefer to place them on the bottom of the
tank and then place a piece of shadecloth between the light
and the new coral. This means that only the new coral is affected
by the lighting change. After a week I remove the shadecloth.
Later, I move the coral to a place closer to the lights, and
then eventually to its final position.
Some corals may have come from lighting conditions similar
to those in the tank, however, as the coral may be stressed,
reducing the irradiance the coral receives is wise, at least
until the coral has had a change to settle in. Exposing corals
to lower light for these short times will have no detrimental
effect. Reducing the photoperiod will only provide some benefit
because the amount of time for damage to the corals to occur
is quite short.
Some hobbyists don't bother photoacclimating their new corals
and don't have any problems. It is far safer, however, to
do some photoacclimation, especially if the corals are stressed.
Conclusion
Hopefully, I have shown the benefits
of photoacclimation of corals and that by gradually increasing
the amount of light new corals receive, aquarists will have
few losses of new corals.
|

