|
Introduction
In many instances,
a diagnosis of cancer is no longer the death sentence that
it once was. Astonishing advances in the three classical modes
of treatment (radiation, surgery and chemotherapy), along
with more contemporary approaches such as photodynamic therapy,
offer
new hope to many patients.
Of these distinct forms of medical intervention, perhaps
chemotherapy
holds the most promise for selectively eradicating
cancer cells while at the same time minimizing collateral
damage to surrounding tissues. In a typical chemotherapy regime,
a patient is treated with one or more chemical agents that
either selectively kill cancer cells directly or promote the
death of cancer cells by indirect means, such as disrupting
solid tumors' blood supply network. Many of these chemical
agents owe their origins to natural sources in the environment,
whereas other anticancer chemotherapeutics are wholly designed
by pharmaceutical scientists based upon current knowledge
of cancer onset mechanisms. Selectivity for cancer cell destruction
without harming healthy cells is the central focus of these
treatment protocols, and chemotherapy's well-known side effects
(hair loss, nausea, immunocompromise, etc.) are a continuing
reminder that much room for progress remains.
Whether the promise of fully selective anticancer medicines
will be realized in our lifetime remains unknown, but exciting
developments from the coral reefs and their inhabitants lend
credibility to the proposition that the best is yet to come.
In fact, coral reef chemistry is coming increasingly to the
foreground in the search for new medicines in general, as
the long-held dogma that the tropical rain forests were going
to be the world's pharmacopoeia has not held up to repeated
examination (Cragg, 2005) despite their harboring over half
of the world's 250,000 known
species of plants. The whole concept of prospecting for
pharmaceutical agents in the marine environment was validated
by the isolation and identification of some simple nucleic
acid analogues, called spongothymidine and spongouridine,
from the Caribbean sponge Cryptotethya crypta over
50 years ago (Bergmann and Feeney, 1950, 1951; Bergmann and
Burke, 1955). These (at the time) unprecedented structures
led chemists to think about and then design structurally related
analogues for testing against a range of human diseases, including
cancer and AIDS. Several successful drugs have been introduced
based upon this sponge isolate-inspired research, including
the antileukemic agent ara-C (Upjohn, now Pharmacia), the
antiviral compound ara-A (Burrows Wellcome, now Glaxo SmithKline),
and the first effective treatment against AIDS, AZT. Interestingly,
the wholly synthetic spongothymidine analogue ara-A was subsequently
discovered to be a naturally occurring metabolite of the Mediterranean
gorgonian Eunicella cavolini (Newman and Craig, 2004).
The discovery of potentially life-saving medicines from reef
inhabitants raises profound issues of ecology and economy.
Is it ethical to exploit, perhaps to the point of extinction,
the producing organisms in order to save human lives? The
fragile and deteriorating health of the tropical reefs has
long been documented, and it is arguable that the large-scale
harvesting of sessile organisms might further exacerbate this
decline. Fortunately, the newer technologies of mariculture
and, independently, genetic engineering, may provide a sustainable
solution. As will be documented below, significant quantities
of several anticancer chemotherapeutic lead compounds can
be obtained from organisms raised through mariculture. An
even more appealing but perhaps more distant solution might
stem from the observation that many, and probably most, of
the chemotherapeutic agents derived from marine species are
actually synthesized by symbiotic microorganisms that live
in the host sponge, coral, etc. Culturing these microorganisms
in the laboratory, and/or to cloning the genes responsible
for the biosynthesis of the compound of interest into well-behaved
bacteria, are active and ongoing concerns. These efforts are
at the forefront of marine natural products research, and
they represent the best hope for satisfying the potentially
conflicting goals of saving the reefs and improving human
health. In order to set the stage for the role of reef flora
and fauna in cancer chemotherapy, a brief and (overly) simplified
description of the whole cancer chemotherapeutic enterprise
follows.
Drug Discovery
The development
of new anticancer medicines is an arduous task that requires
the coordinated efforts of teams of scientists from all manner
of chemical and biological sciences. The big issue that must
be solved is selectivity; because cancer cells are just normal
cells whose growth mechanisms have run amok, deliberately
and precisely targeting them for destruction without harming
the similar healthy cells remains a challenging task. Nevertheless,
many exploitable differences distinguish cancer cells from
their non-cancerous siblings, and these differences become
the focal point of efforts to develop effective anticancer
agents. Whereas a detailed discussion of the biochemical basis
for this divergence is beyond the scope of this article, it
is worth noting that differences in blood supply, oxygen content,
DNA access and chemical signaling pathways, among many other
factors, have been identified and exploited in this regard.
Fortunately, this wide range of different behaviors/characteristics
between cancerous and healthy cells ensures that there is
more than one way to attack the problem of selective cytotoxicity
(= cell killing), so many different types of molecules can
be explored for their ability to act as cancer cytotoxins.
Any potential cancer chemotherapeutic candidate
must undergo a rigorous series of tests prior to gaining FDA
approval for commercialization. The process typically starts
with the basic question of whether the molecule will, in fact,
kill cancer cells. These types of assays are commonly performed
in
vitro against a panel of 60 different types of cancer
cells held in a repository at the National Cancer Institute
(NCI). In addition, some mechanisms by which molecules might
kill cancer cells are indirect, so additional testing in whole
animal and/or xenograft
models may be conducted as well. Success at this level
then leads to further in vitro testing of toxicity
against normal cell lines, etc.
The results of preclinical trials with anticancer agents
are commonly given as ED50 values, which is the
effective dose that kills 50% of the cancer
cells. These ED50 values typically are reported
in concentration units: fractions of moles/liter (L), where
1 mole = 6 x 1023 molecules.
The smaller the ED50, the lower the concentration
of compound necessary to cause cancer cell death, and the
more likely that the molecule will remain on track for further
evaluation. An unofficial threshold below which compounds
are considered active, and therefore candidates for further
study, is low micromolar (mM, 1
mM = 10-6
moles/L, or about 1 part-per-million for a typical drug-sized
molecule). If the ED50 > ~ 10 mM,
then it is unlikely that the molecule will be selective enough
for its biological target, compared to other possible interaction
sites, to remain a viable chemotherapeutic candidate. As detailed
further in this article, the anticancer molecules from the
reef environment that have been selected for further development
all have an ED50 in the nanomolar (nM, 1 nM = 10-9
moles/L, or about 1 part-per-billion for a typical drug-sized
molecule) to picomolar (pM, 1 pM = 10-12
moles/L, or about 1 part-per-trillion for a typical drug-sized
molecule) range.
A molecule becomes a candidate for testing in humans if it
displays both toxicity against cancer cells and is tolerated
by healthy cells/whole animals. Human testing is tightly regulated
for ethical reasons and follows a three-phase protocol. Initially,
Phase I tests are conducted. These tests involve treatment
of a small number of healthy (paid) volunteers with the drug
candidate in order to ascertain whether humans tolerate the
compound. If no adverse effects are detected, then Phase II
trials can commence. This part of the drug validation process
recruits a small number of patients with different cancers
that did not respond to other treatments. Overwhelming success
is not expected, because these cancers are typically refractory
and beyond conventional treatment. Nevertheless, any sign
of improvement is encouraging, even if the cancer is not destroyed.
Drug candidates that continue to show therapeutic potential
at this point then enter Phase III trials, in which they are
administered to a broad range of cancer patients. Dosing schedules,
long-term tolerance and therapeutic efficacy are determined
during these trials, which can be quite lengthy. Eventually,
if the drug candidate survives these experimental challenges,
the compiled data are presented to the FDA for evaluation.
Approval from the FDA for commercial sale then leads to a
new anticancer drug on the market. While these human tests
are ongoing, important issues involving pharmacokinetics,
drug delivery methods, allergic reactions, etc. are investigated
as well. The FDA does not play a passive role in this testing
process; rather, it closely monitors progress with the intent
of "fast-tracking" to market any promising candidates.
Some very thorny issues, such as placebo usage to validate
the trials, arise as potentially life-saving drugs are subjected
to these lengthy experiments, and many pressures come to bear
on the process from medical practitioners, patients and the
drug's developer, typically a large pharmaceutical firm.
The statistics of drug discovery are daunting:
- Failure
rate of all preclinical drug candidates: >> 99.9%
- Failure
rate of preclinical anticancer natural product candidates,
through 1981: ~ 96% (Bhakuni, 2005) (natural product = a
discrete chemical that is extracted from a living organism)
- Failure
rate of all Phase I drug candidates: ~ 30%
- Failure
rate of all Phase II drug candidates: ~ 50%
- Failure
rate of all Phase III drug candidates: ~ 50%
- Failure
after marketing: ~ 10% (Boreman, 2006)
- It
costs about $1.2 billion in current dollars to develop a
new drug, from discovery to commercialization (Hileman,
2006).
These compelling numbers make it clear
that a high premium is placed on discovering good lead compounds.
During the period from 1981 - 2002, 79 new anticancer drugs
were brought to market (Newman, 2003). Of these molecules,
30 were extracted from natural sources (or were derived from
molecules extracted from natural sources), often guided by
folklore medicine. In fact, the NCI maintains an ever-expanding
repository of over 60,000 plant and marine organism samples
available for testing, and about 700 new marine samples are
added each year. None of the source organisms for these 79
drugs was of marine origin, reflecting an early bias toward
exploring the more readily accessible terrestrial flora, as
marine collection was, until recently, not very practical.
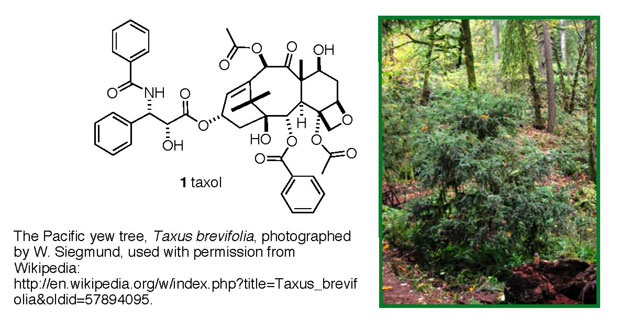
One of the real success stories that has emerged from these
efforts is the anticancer drug taxol (1
trade name: paclitaxel), originally isolated from the bark
of Pacific yew trees, Figure 1. It took over 20 years to shepherd
this compound through all of the isolation, structural/chemical
work and biological testing necessary to gain FDA approval,
but in 1993 it became available to cancer patients. Taxol
is approved for use against ovarian, lung and breast cancers,
and Kaposi's sarcoma. Since its release to the public, it
remains the top-selling anticancer drug, with maximum annual
sales of ~ $1.6 billion in 2000 and $0.9 billion in 2004.
 |
|
Figure 1. Taxol (1) and its source, the
pacific yew tree.
|
Throughout this article the chemical structures of the anticancer
compounds under discussion will be provided in a pictorial
representation that typically is used by organic chemists.
The "language" of organic chemistry, like any language,
has its own self-consistent rules of grammar, sentence structure,
shortcuts and iconography. It is beyond the scope of this
article to delve any more deeply into these details, but the
take-home messages from these molecule depictions are (1)
they all are complex with many atoms, and many connections
(bonds) between atoms, and (2) they all are very different
from one another. They are but a glimpse into the whole portfolio
of potentially active drug candidates, and elucidating the
relationship between the intimate details of their molecular
structure and their observed anticancer activity occupies
a good deal of pharmaceutical research. The premise behind
that research is that if a set of "rules" that relate
structure to function for any given compound can be identified,
then that information might aid in the de novo design
of new drugs for that disease, but perhaps with advantages
not available to naturally derived compounds. It is this goal
that drives much of the pharmaceutical industry.
Where will the next taxol come from? That is the pivotal
question which commands the attention of thousands of pharmaceutical
scientists, oncologists and medical researchers. The attractiveness
of natural sources for anticancer medicines remains high for
a variety of reasons, but the bottom line is a statistical
argument: if all of the matter in the universe were converted
to random, different and unique drug-like molecules, there
still would not be enough molecules to guarantee that an active
drug (for any disease) would be in hand -too many structural
variables are involved. This sobering conclusion comes from
a comparison of the estimated mass
of the universe (~3 x 1055
gm) to the most commonly cited calculated number of conceivable
drug-like molecular entities (>1060
different and discrete chemical structures that have the characteristics
commonly
found in drug molecules). So, the question arises, how
can these infinitesimal odds for finding a drug be improved?
If screening random molecules is futile, might any sources
of molecules be inherently biased toward desirable biological
activity? Many researchers in this area feel that the best
bet lies with molecules derived from natural sources ("natural
products"), because natural products' structures and
chemistries have evolved over geological time to serve as
effective partners for protein receptors, the key molecular-level
event that determines a molecule's potential to act as a drug
(Williams, 1989).
One starting point is simply observing organisms in their
natural habitats in order to discern behaviors that might
signal the production of (cyto)toxic materials. Immobile organisms
(e.g., plants, sessile marine invertebrates) communicate via
chemicals, and the messages that they send and receive involve
all forms of behavior, from mating calls to defense against
predation to attacking/subduing prey or competitors. Chemists
screening for potential anticancer agents take special note
of messages that are lethal to their recipients. If an organism
has evolved an effective cytotoxin, presumably to use against
another organism in its environment, could that molecule be
co-opted to serve as a selectively lethal agent against cancer
cells? These observations provide an enormous head start on
the road to drug discovery.
The marine environment offers drug hunters some attractive
features not shared by terrestrial locations. First and foremost
is its species diversity. It has been estimated that between
one million and two million different species of (mostly microbial)
organisms live in the marine environment. Almost all of these
species are concentrated in either the ~ 1% of fringe territory
between sea and land where reefs abound, or near deep sea
thermal vents (Simmons, 2005). Proximity fosters spatial competition,
especially for living space among sessile invertebrates. That
inevitability apparently has led to the development of sophisticated
chemical arsenals in many of these organisms. The second feature
of marine organisms that recommends them for drug prospecting
is the generally high potency of their chemical agents. Unlike
their terrestrial counterparts, marine organisms must overcome
the enormous dilution factors involved when dispersing chemicals
into the sea. These chemical agents must be effective at the
very low concentrations that dilution by seawater imposes.
Consequently, some of the most potent cytotoxins known have
been found in extracts from marine organisms. Through 2004,
approximately 16,000 discrete chemical compounds had been
isolated and characterized from marine sources (Bhakuni, 2005),
although only a fraction of these species have been subjected
to detailed biological evaluation as chemotherapeutic agents
for any disease.
At present, about 22 marine-derived natural products are
in either Phase I, Phase II or Phase III clinical trials for
anticancer efficacy (Newman, 2006). A description of four
promising candidates follows, and in addition, mention is
made of an interesting soft coral-derived preclinical candidate
whose study has directly benefited from the marine ornamental
(i.e., reef hobbyist) trade.
Ecteinascidin 743
Ecteinascidin 743 (2)
was isolated from the tunicate, Ecteinascidia turbinata
(often called a "sea squirt"), a species of soft-bodied
filter feeder found in the Caribbean and Mediterranean Seas,
Figure 2 (Cragg, 2005; see also Newman, 2006a). In what is
to become a common theme in the discussion of producing organisms,
further scrutiny of the tunicate revealed that, in fact, a
symbiotic microorganism, Endoecteinascidia frumentensis,
appeared to be the actual source of ecteinascidin 743.
Preliminary ecteinascidin 743 in vitro screening experiments
against ovarian, breast and non-small cell lung cancer cell
lines revealed an astonishing potency, down to the picomolar
range, or about 0.7 parts-per-trillion! Follow-up Phase I
trials identified tolerable dose regimens for application
to human cancers, and both Phase II and Phase III trails currently
are being pursued. In total, approximately 2000 cancer patients
have been treated with ecteinascidin 743, and the most promising
results have emerged from both ovarian cancer and soft tissue
sarcoma (STS) studies. In one trial of ovarian cancer patients
(overall five-year survival rate ~ 20%), 10 of 29 patients
(35%) exhibited tumor shrinkage with no recurrence at the
six-month mark. Soft tissue sarcomas are aggressive cancers
with bleak long-term prognoses (overall five-year survival
rate ~ 8%). Treating a group of 183 STS patients with ecteinascidin
743 led to the following promising responses: 14 experienced
tumor shrinkage greater than 50%, 14 achieved tumor shrinkage
of 25 - 50 %, and 66 exhibited tumor stabilization (neither
growth nor shrinkage). These encouraging results place ecteinascidin
743 at the forefront of anticancer agents derived from marine
organisms, and approval for commercialization by Ortho Biotech
(Johnson & Johnson) is expected sometime in 2006. However,
it would not be practical to harvest the producing organism
for the drug, given the quantities necessary for broad treatment,
as one ton of tunicates (about the weight of an American bison
("buffalo")) would yield only 1 gram of compound
(about the weight of a packet of artificial sweetener). As
an alternative, chemists have devised an industrial-scale
synthesis of this complex molecular architecture starting
from a readily available and structurally-related bacterial
fermentation product called saframycin.
|
|
|
Figure 2. Ecteinascidin 743 (2) and its
nominal source, the tunicate Ecteinascidia turbinata.
|
The biochemical mechanism by which ecteinascidin 743 selectively
kills tumor cells is a subject of much active research, and
that story is far from complete. It appears to react chemically
with certain DNA segments, and through these interactions,
disrupts the normal biochemical machinery of DNA reading and
repair. The specific genes that ecteinascidin 743 attacks
code for proteins that regulate several aspects of cell division,
but the remaining cellular DNA does not appear to be otherwise
affected. In addition, ecteinascidin 743 interferes with DNA
repair mechanisms, another potentially lethal action. The
basis for this fortuitous DNA preference remains unclear,
but it can be exploited to terminate cancer cells with sufficient
selectivity to qualify ecteinascidin 743 as a useful drug.
Auristatin
Auristatin (3),
a wholly synthetic analogue of the naturally occurring marine
isolate dolastatin 10 (4), differs
from the natural material only by deletion of the green
thiazole unit in 4 (Figure 3) (Cragg,
2005). Dolastatin 10 was originally isolated from the Indian
Ocean sea hare, Dolabella auricularia, a common algae
eater that is broadly available in the marine aquarium trade
(Figure 3). Subsequent studies revealed that the sea hare
does not actually make the dolastatins (over a dozen related
species have been identified to date), but rather simply sequesters
them from its diet, presumably to act as part of its defensive
arsenal. The actual source of the dolastatins appears to be
the cyanobacteria upon which Dolabella grazes (i.e.,
Symploca sp. VP642, Symploca hynoides and Lyngbya
majuscule), and several of the dolastatins have been isolated
from these primary sources. The amount of dolastatins recoverable
from the sea hares is minuscule: about 10 milligrams (~ the
weight of an uncooked grain of rice) of dolastatin 10 from
10,000 sea hares, and so total chemical synthesis, both of
the natural products and of the more promising analogues such
as auristatin, is employed to provide drugs for testing.
|
|
|
Figure 3. Auristatin (3), dolastatin 10
(4), and dolastatin 10's nominal source, the
sea hare Dolabella auricularia.
|
Preliminary in vitro screens against various cancer
cell lines revealed remarkably potent cytotoxicity for dolastatin
10, much like ecteinascidin 743, down at the picomolar level
(~1 part-per-trillion), against ovarian cancer, non-small
cell lung cancer and myeloid leukemia, among others. Followup
toxicology and Phase II studies provided only disappointment,
as it appeared that problems with the agent's overall toxicity
and, as a consequence, its insignificant anti-tumor effects
at tolerable doses, rendered it unsuitable for further development.
However, the dolastatins' modular structure (a linear sequence
of amino acids) facilitated the exploration of structural
modifications that might preserve anticancer activity but
suppress systemic cytotoxicity. One such analogue is auristatin.
It displayed less in vitro potency than dolastatin
10, acting at about the 1 part-per-billion level against non-small
cell lung cancer, but it did not carry the burden of systemic
cytotoxicity that derailed the dolastatin 10 drug development
effort. Phase I trials for auristatin have been completed,
and this anticancer agent is currently in Phase II, with Phase
III trials to start soon. Data in the public literature for
auristatin are limited, but one report does indicate that
4 of 44 patients with non-small cell lung cancer experienced
complete or partial responses upon exposure to this drug candidate.
The biochemical mechanism-of-action for auristatin and the
dolastatins is quite different from that of ecteinascidin
743, as these Dolabella-derived agents do not act on
a cell's DNA. Rather, they appear to interact with components
of the cellular scaffolding that is required for successful
cell division. This interaction disrupts the normal processes
of cell division, and ultimately the cell dies. The details
of these interactions are still under active investigation.
The basis for cancer cell-over-healthy cell selectivity is
not known, but it may be no more complex than the fact that
cancer cells are continuously dividing and are therefore susceptible
to interference with their division mechanism, whereas normal
cells are mostly quiescent.
E7389
E7389 (5)
is the common name given to a synthesized derivative of the
sponge isolate halichondrin B (6)
(Figure 4) (Cragg, 2005, and Newman, 2006b). E7389 exhibits
an anticancer profile similar to that of the parent 6,
but E7389's greatly simplified structure (the green
half of halichondrin B has been deleted) renders it a far
more attractive candidate for drug development. Halichondrin
B and structurally related congeners were isolated from several
species of sponges, including Halichondria okadai from
the waters of Japan, an Axinella sp. from the western
Pacific, Phakellia carteri from the eastern Indian
Ocean and Lisodendoryx sp. from deep water off New
Zealand's coast. The results of initial cytotoxicity screens
generated enough excitement about halichondrin B's potential
as an anticancer agent that a massive collection program was
conducted. This effort yielded about 300 milligrams of 6
(about the weight of an aspirin tablet) from 2200 lbs. of
Lissodendoryx (about the weight of a Toyota Spyder sports
car) harvested around New Zealand. Later in these studies,
it was discovered that this sponge could be aquacultured in
less than 30 feet of water with good success, and with comparable
amounts of halichondrin B present.
|
|
|
Figure 4. E7389 (5),
halichondrin B (6), and halichondrin B's nominal
source, a sponge of the genus Lissodendoryx.
|
The whole relationship between marine sponges, potential
pharmaceutical agents and the reefkeeping hobby deserves more
comment. Sponges appear to be the real natural product factories
of the marine environment in terms of the sheer volume and
structural diversity of the natural products obtained. In
reality, many recent studies have revealed that the sponges
themselves likely contribute little chemistry to the assembly
of these compounds; rather, the sponges are basically repositories
for many species of symbiotic bacteria and other microflora
that actually perform the biosynthesis of the isolated chemical
species. In fact, the microfloral content of many sponges
constitutes more than half the sponge's dry weight (Piel,
2004)! The observation that halichondrin B can be found in
sponges from so many geographically disparate locations is
consistent with the speculation that a microorganism living
within the different sponges, and shared among them, is responsible
for its biosynthesis. No tests of this hypothesis have appeared
yet. In terms of the current state and future directions of
marine natural products chemistry, sponges, or, more precisely,
the microorganisms that cohabitate within sponges, appear
to be the clear center of attention. Sponge collection can
be a chancy endeavor, with issues of sustainability, identification
and access (a political problem) looming large. As an alternative,
the aquaculturing of sponges as a deliberate means to harvest
their natural products' bounty is still in its infancy. It
is in this area that reefkeeping hobbyists are poised to have
a major impact. The argument can be made that the field of
sponge husbandry resides currently where small-polyp stony
coral husbandry stood 20 or so years ago. Some sponges are
as colorful and articulated as the most coveted stony corals,
but knowledge of their care and survivability in reef tanks
remains underdeveloped (Shimek, 2005). If members of the reefkeeping
hobby who are seeking a challenge turn their attention to
identifying conditions under which various sponges can survive
importation, propagation and life in a reef tank in general,
then perhaps the major importers and marine ornamental sellers
will start stocking and selling them in large numbers. In
turn, the lessons gained in sponge husbandry could have a
significant impact on their availability, especially of those
that have been raised under controlled environments. These
types of advances in sponge husbandry can only accelerate
the search for sponge-derived natural products that may provide
pharmaceutical leads. If you cultivate sponges, then the next
blockbuster drug may be residing in your tank even as you
read this!
Back to halichondrin: Sub-nanomolar (~ parts-per-billion)
in vitro potency against a variety of cancer cell lines,
including non-small cell lung cancer, marked halichondrin
B as a candidate for clinical testing (Newman, 2004). However,
a lack of sufficient material thwarted plans to pursue these
studies further. Fortunately, at about the same time that
the preliminary biological activity was documented, chemical
synthesis efforts by Yoshito Kishi at Harvard University led
to the introduction of several halichondrin B analogues, one
of which, labeled E7389, showed very promising activity. Sub-picomolar
(~ parts-per-trillion) in vitro cytotoxicity against
non-small cell lung cancer and low-nanomolar efficacy against
a colon cancer cell line suggested that truncating the halichondrin
B molecule (cf. 5 vs. 6)
did not attenuate its anticancer properties. Sufficient quantities
of 5 were available through chemical
synthesis to continue into clinical trials, and Phase I results
demonstrated good tolerance for this species. Phase II trials
are currently ongoing as a joint venture between the National
Cancer Institute and Eisai Co., Ltd. for both breast and lung
cancer (Newman, 2006a), but results have not yet been reported
in the open literature.
The biochemical mechanism by which halichondrin B and, presumably,
E7389 contributes to cancer cell death has been studied in
detail (Cragg, 2005). The evidence accumulated so far points
to a scenario related to that described for auristatin whereby
6 (or 5)
selectively binds to the components that make up the intracellular
scaffolding erected during cell division, and this binding
disrupts the scaffold's assembly process and hence the normal
course of cell division. The (rapidly dividing) cells so challenged
cannot cope with the disruption and die.
Bryostatin
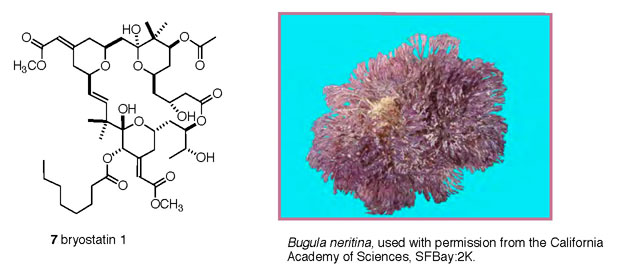
Bryostatin 1 (7)
is representative of a family of 20 structurally related isolates
from the bryozoan (Shimek, 2003) Bugula neritina (Figure
5) (Cragg, 2005). Bryostatins were among the first marine
isolates to demonstrate significant anticancer activity, and
this family of compounds has been under active study for over
30 years. Their promising initial responses in early anticancer
screens led to large-scale collections to compensate for the
exceedingly low amount of material that could be culled from
each organism (< 10 milligrams, at best, from a kilogram
of B. neritina). For example, 28,600 pounds (~ the
weight of the M113 armored personnel carrier) of Bugula
neritina were collected and processed to yield a total
of 18 grams (~ one rounded tablespoon of sugar) of bryostatin
1 - enough for clinical trials to commence. This level of
collection was not sustainable, so the NCI sponsored aquaculturing
efforts that led to successes for both in-sea (mariculture)
and on-land (aquaculture) cultivation of this bryozoan. As
with the previously described cases, the wide geographical
spread of bryostatin-producing B. neritina and the
common observation that many colonies yield no bryostatin
led researchers to speculate that a commensal microorganism,
and not the bryozoan itself, was responsible for the production
of the bryostatins. Subsequent direct probing of this question
led to the discovery that a symbiotic organism, named Candidatus
Endobugula neritina, actually biosynthesizes the bryostatins.
This observation bears some significance to the question of
large-scale bryostatin manufacture should it become a widely
used anticancer drug. At present, it is quite difficult to
identify conditions that would permit cultivation and harvesting
of whole marine invertebrates such as Bugula neritina on a
scale commensurate with commercial needs. However, using microorganisms
as chemical factories has had a long and successful history
in the pharmaceutical industry. Therefore, either culturing
the producing microbe Candidatus Endobugula neritina
itself, or transferring its bryostatin-producing gene cluster
into a well-behaved surrogate microbe, may become viable options
for the commercial manufacture of bryostatin 1.
 |
|
Figure 5. Bryostatin 1 (7), and its nominal
source, the bryozoan Bugula neritina.
|
The preliminary preclinical in vitro data for bryostatin
1 were very promising, and the availability of the material
as described above paved the way for clinical trials. Over
80 human clinical trials have been conducted to date, and
the upshot of these studies is that bryostatin 1 is not a
very effective anticancer agent when used on its own. However,
Phase 1 and Phase II trials that tested bryostatin 1 in conjunction
with another established anticancer agent such as taxol (1)
yielded much more promising findings. For example, 7 of 11
non-small cell lung cancer patients responded favorably to
this combination therapy, whereas in another independent trial,
treatment of chronic lymphocytic leukemia and non-Hodgkin's
lymphoma patients with bryostatin 1 and the anticancer drug
fludarabine resulted in 23 objective responses from a 53-patient
cohort. Currently, Phase II trials for bryostatin 1, in combination
with other established anticancer drugs, are ongoing.
Bryostatin's biological mechanism-of-action is yet again
different from those discussed with the other anticancer compounds.
It selectively binds to, and thereby inhibits the function
of, a critical cell growth enzyme called Protein Kinase C-alpha
(PKC-a). The precise mechanism
by which the down-regulation of PKC-a
activity leads to cancer cell death remains to be unraveled.
Eleutherobin
Corals, especially soft-bodied corals,
are legitimate sources of many secondary metabolites that
display intriguing biological activities. Unfortunately, the
subset of coral metabolites that exhibit significant anticancer
activity on the order of the compounds described above is
minimal at present. Eleutherobin (8)
(Figure 6) is one of the more promising candidates from this
small pool. It was originally isolated from the octocoral
Eleutherobia sp. in Australian waters, and its preliminary
anticancer screens were encouraging. Further collection was
disallowed for political reasons, so the problem languished
until the encrusting Caribbean gorgonian Erythropodium
caribaeorum was found to provide eleutherobin at the 10
milligram-per-kilogram level. Subsequently, material was also
made available through total chemical synthesis. This story
took an interesting turn when it was recognized that E.
caribaeorum was "a staple of the decorative seawater
aquarium industry" (Taglialatela-Scafati, 2002). The
E. caribaeorum cultured in reef tanks did, in fact, produce
eleutherobin at levels commensurate with those from the wild
coral. In this instance, aquarists really did have a potential
anticancer drug within their domain! Whether eleutherobin
is produced by the coral itself or by a symbiotic microorganism
has not yet been addressed.
 |
|
Figure 6. Eleutherobin (8) and its producing
organism, the encrusting gorgonian Erythropodium
caribaeorum.
|
The initial preclinical screens revealed in vitro activity
at the 10 nM (~15 parts-per-billion) level against several
cancer cell lines (Lindel, 1997). However, no further in
vivo studies have been described in the open literature.
One of the reasons this compound in particular has generated,
and continues to generate, much interest is that it appears
to exert its cytotoxicity through the same molecular mechanism
used by the clinically successful anticancer agent taxol (1).
Eleutherobin binds (competitively with taxol) at the cellular
scaffolding that is assembled during division and disrupts
the whole cell division process, leading to cellular death.
A related mechanism was described earlier for E7389, although
in the case of taxol and eleutherobin, drug binding prevents
the scaffold from disassembling at the appropriate point in
the cell division sequence. With E7389, on the other hand,
the assembly process itself is interrupted.
Conclusion
In summary, very promising anticancer
activity has been documented for several naturally occurring
compounds (or their chemically synthesized analogues) derived
from organisms that inhabit the tropical reefs. Several of
these compounds have advanced to human trials, and the results
remain encouraging. Should any of these compounds move to
commercialization, it is unclear whether large-scale production
hurdles can be overcome. Further expansion of the knowledge
base for aquaculturing the nominal producing organisms can
only help this effort, and it is in this area that reef hobbyists
can conceivably have a favorable impact. In addition, the
near universal conclusion that the natural products of chemotherapeutic
interest are actually biosynthesized by microbial symbionts
raises intriguing questions about the relationship between
the "container" organism and its environment. How/why
does it recruit its microflora, and how/why are the symbionts
stimulated to produce the compounds of interest? Growing these
sessile invertebrates under varying, but controlled, conditions
might be one avenue by which some insight can be gained on
these points. Again, these are questions that could benefit
from the input of the reef aquarium hobby.
|