|
In a previous
article I showed how to make your own inexpensive two-part
calcium and alkalinity supplement system. The only materials
required are calcium chloride (available in bulk as a deicer
for pools, for cement making and even for weighing down tractor
tires), baking soda (from a grocery store) and Epsom Salts
(which are inexpensive and available at most drug stores).
Recently, however, some aquarists have found good quality
bulk sources of magnesium chloride (sold as a deicer by the
Dead Sea Works company). Using magnesium chloride improves
the recipe and eliminates a primary concern with the previous
recipe: the potential buildup of sulfate over time.
This article provides an improved recipe for the two-part
additive using magnesium chloride. This improved recipe does
not substantially skew the aquarium's ionic balance. This
article also provides the original recipe for folks who cannot
find, or choose to not use, magnesium chloride. The buildup
of sulfate over time when using the original recipe is shown
graphically under different water change scenarios.
The sections are:
Introduction
This two-part additive system is similar
to the many commercial two-part additive systems. It allows
aquarists to supplement calcium and alkalinity without greatly
skewing the water's ionic balance (something that is claimed
by many of the commercial products, but that is not independently
verified). Equal addition of the two parts to a reef aquarium
will provide calcium and alkalinity in approximately the same
ratio used in calcification by corals and coralline algae.
One part is calcium chloride dissolved in water, and the
other part is baking soda (either baked or not prior to use)
dissolved in water. The balance between these two additives
is very important, and the recipe is designed for aquarists
to dose equal portions of the two parts every time they dose.
An aquarium using such a balanced additive system is unlikely
to undergo large short-term swings in calcium and alkalinity,
as can happen if an aquarist using independent additives were
to inadvertently overdose one or the other. This problem is
surprisingly common, and using balanced calcium and alkalinity
additive systems for most additions serves to eliminate that
potential danger.
A "third" part of this additive system contains
magnesium, sulfate, and chloride. It needs to be added only
once in a while at a fixed rate relative to the other two
parts. It cannot be readily combined with either of the other
parts, based on the ingredients discussed here that are readily
available to aquarists (commercial systems may have more chemicals
to select from, such as sodium sulfate, allowing more flexibility).
This third part is necessary to prevent magnesium depletion,
and to prevent abnormal chloride and sulfate ratios in the
aquarium.
The seven most abundant ions in seawater, in decreasing order
of concentration, are chloride, sodium, sulfate, magnesium,
calcium, potassium and bicarbonate. Using this new recipe
will keep all of these ions in their appropriate ratios (detailed
below).
Comparing the Two Primary Recipes
This article actually
details two primary recipes. One uses raw baking soda, and
the other uses baking soda that aquarists bake before use.
The baking drives some of the carbon dioxide out of the baking
soda, and raises its pH as well as its alkalinity. Be careful
about substituting other brands for the Dowflake and the magnesium
chloride sold by the Dead Sea Works company. A later section
in this article details substitution issues.
Recipe #1 is for use in reef aquaria whose pH is normal
to low. In practice, more reef aquarists end up choosing this
recipe than Recipe #2. It will tend to raise pH due to its
alkalinity part's elevated pH, as do most of the commercial
two-part additives. The increase in pH depends on the aquarium's
alkalinity and, of course, on how much is added. Adding on
the order of 0.5 meq/L of alkalinity increases the pH by about
0.3 pH units immediately upon its addition (and even higher,
locally, before it has a chance to mix throughout the aquarium).
If you are using limewater (kalkwasser) and the aquarium
is at pH 8.4 or above, this recipe is not the best choice.
Otherwise, it is likely to be a good option. It is twice as
concentrated as Recipe #2, because the baking process makes
the baking soda more soluble.
Recipe #2 is for use in reef aquaria whose pH is on
the high side (above 8.3 or so). It will have a very small
pH lowering effect when initially added. The pH drop achieved
will depend on the aquarium's alkalinity and, of course, on
how much is added. Adding on the order of 0.5 meq/L of alkalinity
drops the pH by about 0.04 pH units immediately upon its addition.
If you are using limewater (kalkwasser) and the aquarium
is at pH 8.4 or above, this recipe may be the best choice.
It is half as concentrated as Recipe #1 because the raw baking
soda is less soluble because it's unbaked.
Recipe #1
In this recipe three stock solutions
are made. Two are used frequently, and one is used only occasionally
to balance other elements not added in the first two. The
solutions can be mixed and stored in any plastic or glass
container, and they will last indefinitely. Plastic 1-gallon
milk jugs (typically made of HDPE, high density polyethylene)
can be a good storage choice.
Recipe # 1, Part 1: The Calcium
Part
Dissolve 500 grams (about 2 ½ cups) of calcium chloride
dihydrate (such as Dowflake
77-80% calcium chloride or ESV
calcium chloride; see below for substitutes and sources) in
enough water to make 1 gallon of total volume. You can dissolve
it in about ½ gallon of water, and then pour that into
the 1 gallon container and fill it to the top with more freshwater.
This solution has about 37,000 ppm calcium.
Figure 1. A bag of Dowflake obtained at a Home Depot
store
in the Boston area. Photo by Moe Kirby.
If you use an anhydrous or monohydrate calcium chloride
(such as Dow
Mini-Pellets, Kent's Turbo Calcium, Prestone Driveway
Heat or Peladow
Calcium Chloride), then you should use about 20% (1/5)
less solid calcium chloride by volume to make the recipe.
Note that the solution will get quite hot when dissolving
anhydrous calcium chloride. See the section on substitutions
for further information.
Figure 2. A container of Peladow obtained at a supply
store
in the Boston area (Amesbury Industrial Supply).
Photo by Patrick Higgins.
Recipe #1, Part 2: The Alkalinity
Part
Spread baking soda (594 grams or about 2 ¼ cups)
on a baking tray and heat in an ordinary oven at 300°F
for one hour to drive off water and carbon dioxide. Overheating
is not a problem, either with higher temperatures or longer
times. Dissolve the residual solid in enough water to make
1 gallon total. This dissolution may require a fair amount
of mixing. Warming it speeds dissolution. This solution will
contain about 1,900 meq/L of alkalinity (5,300 dKH). I prefer
to use baked baking soda rather than washing soda in this
recipe as baking soda from a grocery store is always food
grade, while washing soda may not have the same purity requirements.
Arm & Hammer brand is a fine choice. Be sure to NOT use
baking powder. Baking powder is a different material that
often has phosphate as a main ingredient.
Once these two solutions are created, they can be added as
frequently as necessary to maintain calcium and alkalinity.
For further dosing instructions, see below.
Recipe #1, Part 3: The Magnesium
Portion
The magnesium portion gives us two options, with Part 3A
being preferred from an aquarium chemistry standpoint. Pick
one and follow the same dosing directions regardless of which
version you select.
Recipe #1, Part 3A
Dissolve Epsom salts (3 cups) and magnesium chloride hexahydrate
sold by the Dead Sea Works company (5 cups) in enough purified
freshwater to make 1 gallon total volume. There will likely
be a precipitate that forms even if you fully dissolve both
ingredients separately. That precipitate is calcium sulfate
(calcium as an impurity in the magnesium chloride and sulfate
from the Epsom salts). It is fine and appropriate to dose
the precipitate along with the remainder of the fluid by shaking
it up before dosing.
This solution is added much less frequently than the other
two parts. Each time you finish adding a gallon of both parts
of Recipe #1, add 610 mL (2 ½ cups) of this stock solution.
You can add it all at once or over time as you choose, depending
on the aquarium's size and set up. Add it to a high flow area,
preferably a sump. In a very small aquarium, or one without
a sump, I suggest adding it slowly.
The first time it's added, I recommend adding just a small
portion and making sure there isn't any problem (such as corals
closing up due to stress) before adding the remainder. Make
sure corals and other organisms don't get blasted with locally
high concentrations of the main ingredients or impurities,
or else they may become stressed. This solution contains about
47,000 ppm magnesium, 70,000 ppm sulfate and 86,000 ppm chloride.
Recipe #1, Part 3B
Dissolve a 64-ounce container of Epsom salts (about 8 cups)
in enough purified freshwater to make 1 gallon total volume.
This solution is added much less frequently than the other
two parts. Each time you finish adding a gallon of both parts
of Recipe #1, add 610 mL (2 ½ cups) of this stock solution.
It can be added all at once or over time as you choose, depending
on the aquarium's size and set up. Add it to a high flow area,
preferably a sump. In a very small aquarium, or one without
a sump, I suggest adding it slowly.
The first time it's added, I recommend adding just a small
portion and making sure there isn't any problem (such as corals
closing up due to stress) before adding the remainder. Make
sure corals and other organisms don't get blasted with locally
high concentrations of the main ingredients or impurities,
or else they may become stressed. This solution contains about
47,000 ppm magnesium and 187,000 ppm sulfate.
Recipe #2
In this recipe three stock solutions
are created. Two are used frequently, and one is used only
occasionally to balance other elements not added in the first
two. The solutions can be mixed and stored in any plastic
or glass container. Plastic 1-gallon milk cartons (typically
made of HDPE, high density polyethylene) can be a good storage
choice.
Recipe #2, Part 1: The Calcium
Part
Dissolve 250 grams (about 1 ¼ cups) of calcium chloride
dihydrate (such as Dowflake
77-80% calcium chloride or ESV
calcium chloride; see below for substitutes and sources) in
enough water to make 1 gallon of total volume. You can dissolve
it in about ½ gallon of water, and then pour that into
the 1 gallon container and fill it to the top with more freshwater.
This solution is about 18,500 ppm in calcium. Winn Dixie Ad has new coupons this week again.
If using an anhydrous or monohydrate calcium chloride (such
as Dow
Mini-Pellets, Kent's Turbo Calcium, Prestone Driveway
Heat or Peladow
Calcium Chloride), then about 20% (1/5) less solid calcium
chloride by volume should be used to make the recipe. Note
that the solution will get quite hot when dissolving anhydrous
calcium chloride. See the section on substitutions for further
information.
Recipe #2, Part 2: The Alkalinity
Part
Dissolve 297 grams of baking soda (about 1 1/8 cups) in
enough water to make 1 gallon total. This dissolution may
require a fair amount of mixing. Warming it speeds dissolution.
This solution will contain about 950 meq/L of alkalinity (2660
dKH). As mentioned earlier, Arm & Hammer is a fine brand
of baking soda to use in these recipes. Be sure to NOT use
baking powder. Baking powder is a different material that
often has phosphate as a main ingredient.
Once these two solutions are created, they can be added as
frequently as necessary to maintain calcium and alkalinity.
For further dosing instructions, see below.
Recipe #2, Part 3: The Magnesium
Portion
The magnesium portion again gives us two options, with Part
3A being preferred from an aquarium chemistry standpoint.
Pick one and follow the same dosing directions regardless
of which version you select.
Recipe #2, Part 3A
Dissolve Epsom salts (3 cups) and magnesium chloride hexahydrate
(5 cups) in enough purified freshwater to make 1 gallon total
volume. There will likely be a precipitate that forms even
if you fully dissolve both ingredients separately. That precipitate
is calcium sulfate (calcium as an impurity in the magnesium
chloride and sulfate from the Epsom salts). It is fine and
appropriate to dose the precipitate along with the remainder
of the fluid by shaking it up before dosing.
This solution is added much less frequently than the other
two parts. Each time you finish adding a gallon of both parts
of Recipe #2, add 305 mL (1 ¼ cups) of this stock solution.
You can add it all at once or over time as you choose, depending
on the aquarium's size and set up. Add it to a high flow area,
preferably a sump. In a very small aquarium, or one without
a sump, I suggest adding it slowly.
The first time it's added, I recommend adding just a small
portion and making sure there isn't any problem (such as corals
closing up due to stress) before adding the remainder. Make
sure corals and other organisms don't get blasted with locally
high concentrations of the main ingredients or impurities,
or else they may become stressed. This solution contains about
47,000 ppm magnesium, 70,000 ppm sulfate and 86,000 ppm chloride.
Recipe #2, Part 3B
Dissolve a 64-ounce container of Epsom salts (about 8 cups)
in enough purified fresh water to make 1 gallon total volume.
This solution is added much less frequently than the other
two parts. Each time you finish adding a gallon of both parts
of Recipe #2, add 305 mL (1 ¼ cups) of this stock solution.
You can add it all at once or over time as you choose, depending
on the aquarium's size and set up. Add it to a high flow area,
preferably a sump. In a very small aquarium, or one without
a sump, I suggest adding it slowly.
The first time it's added, I recommend adding just a small
portion and making sure there isn't any problem (such as corals
closing up due to stress) before adding the remainder. Make
sure corals and other organisms don't get blasted with locally
high concentrations of the main ingredients or impurities,
or else they may become stressed. This solution contains about
47,000 ppm magnesium and 187,000 ppm sulfate.
Dosing Instructions
The dosing instructions are basically
the same for each recipe, although any given aquarium will
end up using about twice as much of recipe #2 as recipe #1
to add the same amount of calcium and alkalinity.
To initiate dosing, first adjust calcium and alkalinity to
roughly their correct ranges. This may require a substantial
dose of just the calcium part if calcium is low (e.g., below
380 ppm). I would suggest targeting calcium between 380 and
450 ppm, and alkalinity between 2.5 and 4 meq/L (7-11 dKH;
125-200 ppm calcium carbonate equivalents).
This calculator shows how much of what parts to add in order
to boost one or both of the parameters by a certain amount:
Reef chemicals calculator
http://home.comcast.net/~jdieck1/chem_calc3.html
Then, once things seem roughly correct, select a starting
daily dose for routine dosing. Here are some suggested starting
doses, but the exact values do not matter much. The suggested
doses apply to both recipes.
|
Table
1. Suggested starting daily doses of this supplement
in different aquaria.
|
| Tank
Description: |
Suggested
Starting Doses:
|
| |
Recipe
#1
|
Recipe
#2
|
| Fish-only
with live rock |
0.1
mL/gallon
|
0.2
mL/gallon
|
| New
tank, few corals |
0.2
mL/gallon
|
0.4
mL/gallon
|
| Low
demand |
0.3
mL/gallon
|
0.6
mL/gallon
|
| Mixed
tank |
0.5
mL/gallon
|
1
mL/gallon
|
| Heavy
demand (SPS corals) |
1
mL/gallon
|
2
mL/gallon
|
After a few days of dosing, note whether alkalinity
is low, high or on target. Only bother to test alkalinity,
not calcium, during this period, because it is much more sensitive
than calcium to over- or underdosing. Adjust the dose up or
down as necessary to increase or decrease the alkalinity.
Once you have determined the proper dose, continue it until
there is a substantial reason to adjust it (such as falling
alkalinity as the corals increase in size). When adjusting
the dose, raise or lower both of the recipe's parts together.
Resist the temptation to keep jiggering calcium and alkalinity
independently. They will need occasional corrections, but
that should not be the normal course of dosing unless there
are substantial outside influences, such as water changes
with a salt mix that does not match the tank's parameters
or an error in making the mixes.
Check alkalinity fairly frequently to make sure the dosing
continues at a suitable rate. Check it maybe once a week to
once a month (or less as you get more experienced with the
system and the tank). Check calcium once a month to once every
few months to make sure it continues on track.
Remember to add an appropriate amount of Part 3 each time
you finish adding a gallon of Parts 1 and 2.
Substitutes for Dowflake Calcium
Chloride
If Dowflake calcium chloride or a
repackaged version (such as All-Clear) cannot be located,
Peladow
or Dow
Mini-Pellets, which are dehydrated versions of Dowflake
(that is, they have less water in the crystals), can be substituted.
In addition to the Peladow brand name, Peladow also is sold
as Prestone Driveway Heat and possibly as some other common
brands. Kent Turbo Calcium is also suitable and is an anhydrous
calcium chloride. Any FCC (food), USP (pharmaceutical) or
BP (pharmaceutical) grades of calcium chloride should be suitable.
Peladow, Dow mini-Pellets, Prestone Driveway Heat, Kent Turbo
Calcium and other dehydrated calcium chloride products are
more potent than Dowflake. The dehydration makes them both
more potent by weight, and more dense, so they are much more
potent by volume. The problem is that it is rarely clear how
much moisture they contain. Peladow
specifies 90% calcium chloride minimum, but it may be higher
in some cases. Dow Mini-Pellets say 94% minimum, but it actually
has a lower bulk density than Peladow. The best guess for
an amount to use is based on the hydration levels and bulk
density provided by Dow for these products. Using these numbers,
I suggest that aquarists use 20% less VOLUME of the dehydrated
versions in the recipes than the Dowflake they call for. So
a recipe calling for 5 cups of Dowflake would use 4 cups of
Peladow, Prestone Driveway Heat, Kent Turbo Calcium, etc.
Choosing other unknown brands of any of the products may
be fine, or not. I've not tested them for purity.
Substitutes for Dead Sea Works
Magnesium Chloride Hexahydrate
Dead Sea Works is a business unit
of ICL Fertilizers. They sell magnesium chloride hexahydrate
in the U.S. as a deicer and apparently distribute it also
to artificial seawater (salt) manufacturers. In the past,
potential impurities (such as ammonia) have left many aquarists,
and even some companies, wary of using deicing or any other
grades of magnesium chloride hexahydrate. However, the Dead
Sea Works company recently supplied a detailed
impurity profile of its product listing most impurities
(29 in all). None was high enough to concern reef aquarists.
Included in the profile was an indication that it had adequately
low ammonia. Subsequent analysis by Greg Hiller of some of
the supplied material confirmed that the ammonia is low enough
to use.
The recipe above is based on the MAG Flake's bulk density
supplied by the manufacturer. They also sell a pelletized
product, which may be OK to use, but probably has a slightly
different bulk density (they do not provide the bulk density
for that to my knowledge). Exact values for the magnesium
part are less important than for the other parts, and when
using pellets I recommend just following the directions stated
here for flakes, unless better information becomes available
in the future.
Editors note (3/10/07): Note, the manufacturer of MAG flake has alerted us that they very strongly recommend against using this product in reef aquaria. While many reef aquarists have successfully used the product, the manufacturer does not claim to be able to provide this product at suitable quality in the future. |
Figure 3. One style of bag of magnesium chloride hexahydrate
made by the Dead Sea Works and sold at a supply store in the
Boston
area (Amesbury Industrial Supply). I also saw such a bag at
my local
Home Depot. Photo by Patrick Higgins.
At this time magnesium chloride hexahydrate from the Dead
Sea Works is the only such product that I recommend, but others
may be acceptable. Choosing other unknown brands may be
fine, or not. I've not tested them for purity.
Where to Buy the Materials
Baking soda (sodium bicarbonate) is
best obtained from a grocery store to ensure that it is a
food grade material. Arm & Hammer is a fine brand, as
is a store brand. Be sure to NOT use baking powder. Baking
powder is a different material that often has phosphate as
a main ingredient.
Calcium chloride dihydrate (Dowflake) can often be obtained
at stores such as Home Depot as a deicer. All-Clear calcium
chloride for pools is repackaged Dowflake.
The following links lead to companies that are believed to
supply Dowflake. Some will ship and some may be available
only via local pickup:
http://www.buckeyefieldsupply.com/showproducts...&showspecials=124
http://www.flordrisupply.com/index2.html
http://www.mainstreetseedandsupply.com/saltproducts.htm
http://www.gemplers.com/a/shop/product.asp...=21BR001
http://www.meltsnow.com/products-dry-calcium-chloride.htm
http://www.cal-chlor.com/products.htm
http://www.farrellequipment.com/catalog/ChemicalCementitious.pdf
Peladow is available from some of the suppliers above and
is sold at many home products stores as Prestone Driveway
Heat for deicing.
Magnesium chloride hexahydrate made by the Dead Sea Works
is sold at many home stores, including Home Depot, often labeled
as MAG Flake. It may be repackaged as Meltsnow:
http://www.meltsnow.com/msds-mag-flakes.htm
It may also be available from these stores:
http://www.harveysalt.com/prod01.htm
http://www.meltsnow.com/products-dry-magnesium-chloride.htm
Figure 4. A second style of bag of magnesium chloride
hexahydrate
made by the Dead Sea Works. This packaging was obtained in
a group
buy organized by the Boston Reefers. Photo by Greg Thevenin.
Calculation Rationale for the
Recipes
The calculation rationale that follows
is for Recipe #1. The rationale for Recipe #2 is the same,
except that everything is divided by 2 and baking the baking
soda is not required. This section is provided for those who
want to know how the recipe is devised, who are concerned
that there might be an error or who might want to change it
slightly. It is not necessary to read the following section
if all you want to do is use it.
The Design of the Calcium and
Alkalinity Parts
The Dowflake material is supposed to contain 77-80% calcium
chloride. From the Dow Flake website,
it has a bulk density of 0.82 - 0.96 g/dry mL or 194 - 227
grams/level measuring cup. We will assume that it is 78.5%
calcium chloride by weight and weighs 200 grams per level
measuring cup. Because calcium comprises 36% of calcium chloride,
by weight, each cup contains 200 x 0.785 x 0.36 = 56.5 grams
of calcium.
Consequently, dissolving 2 ½ cups (500 g) of Dowflake
per gallon = 141 grams of calcium per gallon, or 37,300 mg/L.
The final concentration will vary with how much moisture was
actually in the calcium chloride, and how well it packed in
your measuring cup. A concentration of 37,300 ppm calcium
is equivalent to 0.93 molar.
When calcification takes place, two moles of alkalinity are
lost for every one mole of calcium. So, we need to match the
calcium above with 1.86 molar baking soda (sodium bicarbonate)
equivalents (before or after baking, the baking doesn't change
the alkalinity). As I measure it, Arm & Hammer baking
soda weighs about 264 grams per level measuring cup. Because
sodium bicarbonate has a molecular weight of 84 g/mole, we
need to dissolve 1.86 x 84 = 156 grams/L, or about 594 grams
(2 ¼ level measuring cups) of baking soda per gallon.
Note that it doesn't matter how many grams the 594 grams of
baking soda becomes after baking. All baking does is change
the amount of carbon dioxide and water in the baking soda:
2
NaHCO3 à Na2CO3
+ H2O + CO2
More, or less, baking will only alter the pH increase upon
addition to the aquarium. However, substantial under-baking
may make it impossible to fully dissolve the solid material
in the recipe, as sodium bicarbonate is less soluble than
sodium carbonate (which is why Recipe #2 is more dilute).
Overbaking with respect to time or temperature has no negative
effect.
Residual Ions from the Calcium
and Alkalinity Parts
Adding 1 gallon of each of these additives will result in
a residue of ions remaining after calcification. These are
mostly sodium and chloride, and the amounts of those two added
are equal in numbers (i.e., moles), but slightly different
in weight-based concentrations such as ppm because they do
not weigh the same.
After adding 594 grams of baking soda (1 gallon of Recipe
#1), we will have added 163 grams of sodium. In natural seawater,
magnesium is present at about 12.0% of the sodium concentration
(by weight). In order to match the magnesium additions to
the sodium additions to leave them in a natural ratio, we
need to add 12% of 163 grams, or 19.5 grams, of magnesium
for every gallon of the two-part additive that we add.
Additionally, we may want to account for magnesium that is
actually incorporated into the coral skeletons. For this calculation,
I have assumed that the amount of magnesium incorporated is
about 6.5% of the calcium level (by weight), or about 2.5%
of the skeleton by weight. In the course of adding this gallon
of both parts of the two part supplement, we added 141 grams
of calcium, so we need to add 0.065 x 141 = 9 grams of magnesium
to account for this deposition.
The magnesium parts of the recipe are designed to add enough
magnesium so that it is not depleted by either of the two
means described above. Because the magnesium supplement (either
version) is 47,000 mg/L in magnesium, we need to add (9 +19.5)
grams/47 g/L = 610 ml of the magnesium solution for each gallon
of the other parts of Recipe #1.
Interestingly, the potassium
present as an impurity in the Dowflake works to our advantage
in this use. Recipe #1 has 1,342 ppm potassium in its calcium
part. That amount puts it in the right ratio relative to other
ions in the recipe (chloride, sodium, etc.) so that it is
neither boosted nor depleted significantly over time based
on salinity changes (see modeling below).
Residue Remaining from Recipe
#1 when using Recipe #1, Part 3A
After one year of adding 8 ppm of calcium and the accompanying
0.4 meq/L (1.1 dKH) of alkalinity per day (41 mL of both parts
per day or 4 gallons of both parts per year in a 50-gallon
aquarium, including the effect of the magnesium part #3A,
2440 mL/year), the following residue (Table 2) would remain
after calcification and adjustment for salinity (there is
roughly a 32% rise in salinity over a year using this addition
rate without water changes).
Note that in this recipe, all of the ions match NSW fairly
closely (green), but without using Part 3A, the magnesium
and sulfate are severely depleted (red).
|
Table
2. Elements present after one year of additions and
after adjusting for salinity changes. Calculations assume
no water changes take place.
|
| Element |
Seawater
Concentration
|
Final
Tank Concentration (w/ Part 3A)
|
Final
Tank Concentration (w/o Part 3)
|
| Chloride |
19,350
|
19,440
|
19,710
|
| Sodium |
10,760
|
10,730
|
11,360
|
| Sulfate |
2,710
|
2720
|
2170
|
| Magnesium |
1,290
|
1270
|
880
|
| Calcium |
420
|
420
(assumed)
|
420
(assumed)
|
| Potassium |
400
|
384
|
405
|
Residue Remaining from Recipe
#1 when using Recipe #1, Part 3B
After one year of adding 8 ppm of calcium and the accompanying
0.4 meq/L (1.1 dKH) of alkalinity per day (41 mL of both parts
per day or 4 gallons of both parts per year in a 50-gallon
aquarium, including the effect of the magnesium sulfate solution,
2440 mL/year), the following residue (Table 3) would remain
after calcification and adjustment for salinity (there is
roughly a 29% rise in salinity over a year using this addition
rate without water changes):
Note that in this recipe, all of the ions except sulfate
(red) match NSW fairly closely (green), but without using
Part 3A, magnesium and sulfate are severely depleted (red).
|
Table
3. Elements present after one year of additions of Recipe
#1, using Part 3B and after adjusting for salinity changes.
Calculations assume no water changes take place.
|
| Element |
Seawater
Concentration
|
Final
Tank Concentration (w/ Part 3B)
|
Final
Tank Concentration (w/o Part 3)
|
| Chloride |
19,350
|
18,470
|
19,710
|
| Sodium |
10,760
|
10,650
|
11,360
|
| Sulfate |
2,710
|
3840
|
2170
|
| Magnesium |
1,290
|
1282
|
880
|
| Calcium |
420
|
420
(assumed)
|
420
(assumed)
|
| Potassium |
400
|
387
|
405
|
In a previous article discussing water
changes, I showed how the rise in sulfate shown in Table
2 is mitigated to some extent by water changes. Those data
are reproduced in Figure 5 below, which shows the effect of
daily water changes amounting to 7.5%, 15% and 30% on a monthly
basis. Clearly, the 15% and 30% changes per month mitigate
the rise in sulfate over a year by a substantial amount (reducing
the increase by 54% and 74%, respectively).
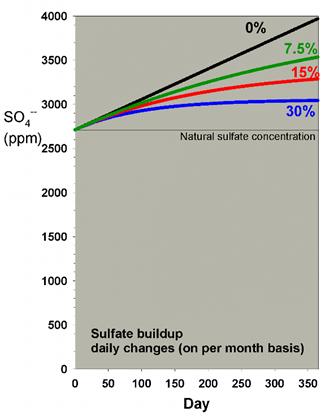 |
Figure 5. Sulfate concentration as a function
of time when performing daily water changes equivalent
to 0% (no changes), 7.5%, 15% and 30% of the total volume
each month (in other words, 0%, 0.25%, 0.5% and 1% per
day). In this example, sulfate starts at a natural level
of 2710 ppm, and the model assumes the usage of a moderate
amount of calcium chloride and sodium bicarbonate to
maintain calcium and alkalinity, and Epsom salts (magnesium
sulfate) to maintain magnesium.
|
Summary
This improved two-part additive system
is inexpensive and simple to make. Many reef aquarists have
been successfully using the original recipe for more than
a year now, and this improvement should make it even more
appealing.
For those who use it, be sure to check the calcium and alkalinity
values over time, even after establishing a routine that looks
to do the job. Because of the uncertainty in the amount of
moisture in these products when purchased, and in the amounts
that you actually measure out, the system may not be perfectly
balanced, and a slow drift toward elevated or depleted calcium
(assuming you are dosing to maintain alkalinity) may take
place even in the absence of other potentially disrupting
factors such as water changes.
Measuring magnesium once in a while is also likely a good
move, just in case it is being used more or less rapidly than
expected for a typical case.
Happy Reefing!
|