|
This article deals with oxygen dynamics in some reef aquaria.
It is the first of what will be a two-part set of articles,
not only because of the length and breadth of material covered,
but also because I have not yet finished collecting and analyzing
all of the data for the second article. I began to think about
oxygen in reef aquaria quite some time ago and only recently
began to explore it in some depth. I have been investigating
the possibility of hypoxia
acting as a trigger for cellular death in corals in my own
work. During the course of background searches and the use
of a chambered aquarium I designed to test hypotheses regarding
the effects of hypoxia on corals, I became interested in the
issues from an aquarist's viewpoint.
Certainly there has been some discussion regarding oxygen
in aquaria over the years. During a recent trip to Atlanta
for Saltwater U. (www.saltwateru.com),
my hosts generously gave me a set of antique aquarium magazines.
In the September 1932 issue of The Aquarium, an article
was devoted solely to the topic of oxygen in aquaria (Timm,
1932). Typically, the first time aquarists become concerned
with oxygen is when shipping and transporting fish from either
their home on tropical reefs, or from a livestock vendor.
Packing fish and coral in bags with a topping of pure oxygen
is testimony to the concern with oxygen levels in small volumes
of water. If fish are packed with only air, the usual drill
is to drive home as quickly as possible to get the new stock
into quarantine or an aerated tank as quickly as possible.
This is somewhat less of a concern with corals and other invertebrates,
but the situation remains similar. Those who have tried to
ship livestock without a topping of pure oxygen will recognize
the disturbingly high mortality that can occur during transit.
In terms of aquariums, I had always heard that using air
bubblers kept tank water oxygenated. I believed it, too, until
someone pointed out that the diffusion across an air bubble
as it rises to the surface and breaks is so low as to be negligible.
It has also been pointed out that most of the gas exchange
occurs across the water's surface, and bubbling or any type
of surface stirring that mixes the water and creates a greater
surface area for gas exchange is far more effective than merely
bubbling air into a tank using an airstone and air pump. Many
aquarists also have stated that protein skimming dramatically
increases "aeration," and that low oxygen levels
in highly skimmed reef aquaria are rarely a concern. I was
always unsure how much oxygenation occurred in a closed chamber
with most of the bubbles being forced to the surface prior
to their return to the tank. Still, it made sense, too, that
water in contact with such a frothy mix must certainly gain
oxygen during its pass through a foam fractionation device.
In fact, so much banter exists around this topic that I began
to wonder if anyone had really measured reef tanks' oxygen
levels, or if any data really existed to support any of the
statements commonly made and accepted.
The Oxygen Environment of Earth
Complex life on our fragile planet Earth would probably cease
to exist, or may never have come to exist at all, without
oxygen. About two billion years ago, a significant buildup
of oxygen began to occur after life first arose approximately
3.85 billion years ago. This buildup of oxygen was largely
due to primitive photosynthetic cyanobacteria. A period of
intense diversification and distribution, known as the Cambrian
Explosion, began 542-544 million years ago when the Earth
contained a level of oxygen roughly equivalent to today's
atmosphere, representing approximately 21% of the gases present.
Bacteria were probably the first life forms. Since bacteria
can be anaerobic or chemoautotrophic [def = being autotrophic
and oxidizing an inorganic compound as a source of energy],
many do not require oxygen at all and to some bacteria oxygen
is a death sentence. In the early years of earth's history,
there was no oxygen. Without oxygen, there is no ozone. Without
ozone, high levels of UV radiation, a potent mutagen,
would strike the planet. At some point, it is believed that
a mutation for oxygen tolerance arose in the archaebacteria
that allowed for the evolution of cyanobacteria, sometime
in the first billion years of life. The cyanobacteria were
able to take up water and release oxygen as a metabolic waste
product.
As oxygen levels rose in a previously anaerobic world, adaptations
had to occur in order for the anaerobes to persist in aerobic
environments. At some point, a bacterium engulfed another
bacterium, and the relationship became one of symbiosis. The
primitive relationship eventually led to eukaryotic life and
the engulfed microbe became the oxygen-utilizing powerplant
of cells, the mitochondrion. Mitochondria still retain their
own DNA, even after a billion years of evolution. The choroplasts
of plants are also a result of an early invasion and subsequent
symbiosis that allowed for photosynthesis.
In time, oxygen levels in the oceans rose, as gases diffused
between air and liquid. This happened very slowly, and primarily
only after all of the exposed rock on the planet was oxidized.
The oceans are still not oxygen-saturated and there is a lot
of evidence that until the mid to late Mesozoic they were
only oxygenated to a depth of a couple of hundred meters.
Liquids, including water with its relatively high solubility,
can, however, dissolve only so much gas. Compared to the atmosphere,
seawater is oxygen-poor.
Oxygen in the Oceans
In the oceans, oxygen
exists in seawater as a result of exchange at the air/water
interface and due to photosynthesis (primarily by marine plants,
algae and phytoplankton). The measure of primary productivity
is roughly approximate to photosynthesis, and is measured
in gC/m2/yr
(where C = carbon). However, direct measurements of
photosynthesis are often taken by measuring in the currency
of oxygen using bottles that are either exposed to light or
left in the dark. This is important, because what such experiments
measure are the relative rates of respiration versus photosynthesis.
The former consumes oxygen; the latter produces it. This small-scale
measurement is a microcosm of what happens in the ocean.
The amount of gas that seawater will hold is a reflection
of several physico-chemical factors. The first is the partial
pressures of the gases in the atmosphere (largely a function
of atmospheric pressure), which is roughly 14.7 psi (760 Torr)
at sea level. Oxygen partial pressure at sea level is roughly
150 Torr. The second factor is temperature; solubility of
oxygen is inversely proportional to temperature. Cooler water
can dissolve more oxygen than warmer water, a fact that is
occasionally mentioned when discussing optimal temperatures
for aquaria. A third factor involves salinity, again representing
an inverse relationship. Seawater of lower salinity can hold
more oxygen than a similar seawater sample of higher salinity.
This attribute is the longstanding rationale for keeping marine
fish in water with a lower salinity than seawater (e.g., 29-30
ppt or 1.022 SG), the argument being that reduced salinity
makes it "easier for the fish to breathe." This
argument, while perhaps true in terms of oxygen, is preposterous.
Marine fish, while some are tolerant of variable salinity,
have evolved to exist in a marine environment where the salinity
is usually between 34-36ppt. Long-term exposure to hyposalinity
may have negative effects that outweigh the benefits afforded
by increased oxygen solubility, a point which will become
increasingly apparent throughout this and the next article.
A fourth factor is pressure, with deep water under enormous
pressure able to dissolve more gas than surface waters. Since
most of our coral reef species are from relatively shallow
waters, and our aquaria are hardly under great pressure (though
our floorboards might be), this factor may be less important
in aquaria than in the ocean.
|
Salinity
(ppt)
|
|
Temp
(°C)
|
0
|
5
|
10
|
15
|
20
|
25
|
30
|
35
|
40
|
|
5
|
14.8
|
14.4
|
13.9
|
13.5
|
13.0
|
12.5
|
12.1
|
11.6
|
11.2
|
|
10
|
13.0
|
12.6
|
12.2
|
11.8
|
11.4
|
11.0
|
10.6
|
10.2
|
9.8
|
|
15
|
10.3
|
10.0
|
9.7
|
9.4
|
9.2
|
8.9
|
8.6
|
8.3
|
8.1
|
|
20
|
9.4
|
9.1
|
8.8
|
8.6
|
8.4
|
8.1
|
7.9
|
7.6
|
7.4
|
|
25
|
8.5
|
8.3
|
8.0
|
7.8
|
7.6
|
7.4
|
7.2
|
6.9
|
6,7
|
|
30
|
7.8
|
7,6
|
7.4
|
7.2
|
7.0
|
6.8
|
6.6
|
6.4
|
6.2
|
|
|
Table 1. Oxygen saturation levels in water (mg/l)
at different salinities and temperatures (from Adey
and Loveland, 1991). Highlighted cells represent those
parameters representative of most reef aquaria.
|
Other factors influence seawater's oxygen content. The amount
of gas exchange occurring at the air/water surface interface
is contingent on the properties of both air and water. Air
circulation correlates positively with increased dissolution,
especially at the surface. Wind, for example, creates higher
oxygen values in surface waters than occur during periods
of calm weather. Water circulation is equally important, and
the mixing that occurs involves waves, currents, upwellings
and circulation patterns. Another factor, perhaps obviously,
is the production of oxygen by photosynthesis, which is now
viewed as a major contributor to oxygen saturation states
in the waters of the photic
zone. Obviously, respiration is also important in terms of
oxygen consumption. The depth where oxygen consumption by
respiration equals oxygen production by photosynthesis is
called the compensation depth. Because of mixing, there is
a depth slightly below the compensation depth, called the
critical depth, where total plant production equals total
plant respiration. Below this depth is the aphotic
zone, where there can still be abundant life despite the lack
of photosynthesis. Animals, such as fishes and plankton, may
vertically migrate seasonally or diurnally to take advantage
of the productive areas above them.
Below the critical depth, however, oxygen levels continue
to drop until a point is reached at approximately 500-1000m
called the oxygen minimum layer, or oxygen minimum zone. Here,
oxygen levels are at their lowest as the organisms are respiring,
exhausting the oxygen replaced by air and photosynthesis.
Some organisms do live in the oxygen minimum zone, although
they have evolved special adaptations to exist in the hypoxic
conditions found there. Below the oxygen minimum zone and
including the very deepest depths, oxygen levels again increase.
This increase, sometimes reaching saturation but rarely reaching
levels present in the shallow photic zone, occurs because
of the cold temperatures and the relatively low density of
deep-sea organisms respiring in the vast volume of deep ocean
water. The deep sea's oxygen is provided mainly by downwelling
currents near the poles where cold surface water sinks to
the deep ocean where there is less mixing.
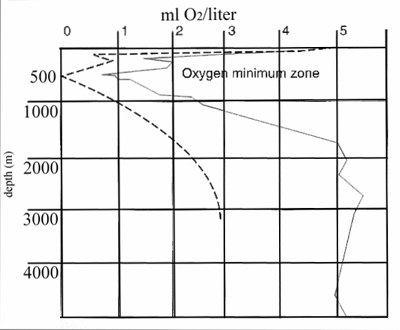 |
|
Figure 1. The oxygen minimum zone of the tropical
eastern Pacific (dashed line) and tropical Atlantic
(solid line), modified from Nybakken (2001).
|
Shallow coral reef waters are often supersaturated with
oxygen. This is because of the high productivity resulting
from algal and coral photosynthesis, coupled with shallow
water and often-strong tropical air and water circulation
patterns. Despite the general notion that shallow tropical
waters are well oxygenated, it has long been known that areas
of lower oxygen exist, especially where there is comparatively
little water exchange, such as lagoons, some reef flats cut
off from circulation at low tide, and even shallow reefs,
in general, during periods of doldrums.
Oxygen and Coral Reef Fishes
In one of the more interesting studies
I have found on the subject, Nilsson et al. (2004) examined
hypoxia tolerance in the coral-dwelling goby, Gobiodon
histrio. More on G. histrio can be found in another
article
in this magazine by Henry Schultz. This species spends its
whole life inside branches of Acropora, showing a preference
for A. nasuta. The authors suspected that the oxygen
environment of the coral could be highly variable, being exposed
to air at low tide, and also possibly becoming hypoxic at
night. They specifically mention calm nights when the respiration
of the coral and associated organisms and lack of water mixing
created a hypoxic environment for the goby (discussed in more
detail below). In fact, they found that such conditions did
exist and that G. histrio was extremely hypoxia tolerant,
only showing equilibrium loss at water oxygen levels of approximately
3% of air saturation.
Nilsson and Östlund-Nilsson (2004) expanded this report
by examining hypoxia tolerance in 31 species (7 families)
of fish in the shallow lagoon of Lizard Island on the Great
Barrier Reef. They found that hypoxia tolerance was variable
but widespread, with all species maintaining normal respiration
rates in water down to 20-30% of air saturation levels, with
most species unaffected until about 10% of air saturation
levels. In an earlier work, they had found two species of
cardinalfish (Apogon leptacanthus and A. fragilis)
to have a critical oxygen level of approximately 20% of air
saturation, at which point they begin to rely on anaerobic
metabolism. The effect on mouth-brooding and on cardinalfish
is expanded in Östlund-Nilsson and Nilsson (2004). Aquarists
who are breeding or considering breeding cardinalfish should
read that article, and perhaps pay attention to this article
and next month's article, as well. Blennies and gobies, in
general, were the most tolerant of hypoxia. Surprisingly,
damselfishes were a mixed lot, with some showing much higher
tolerance than others. Some damselfish were more sensitive
than even the cardinalfish to hypoxia. It should be noted
that this study used small species or those that seek refuge
within corals or the coral reef framework at night. No mention
is made of fishes that tend to remain in more well-oxygenated
waters at night, most notably for aquarists, the surgeonfishes.
The general groups examined were the cardinalfish (9 species),
the damselfish (14 species), the gobies (3 species), the blennies
(2 species), the filefish (one species), the breams (1 species)
and the wrasses (2 species). Hypoxia tolerance has also been
found in the epaulette shark (Routley et al. 2002).
Oxygen and Corals
The investigation of hypoxia and corals
is only slightly greater in scope than that of fishes, and
is also comparatively recent. As students of coral reef literature
may find unsurprising, C.M. Yonge investigated this aspect
of coral physiology in the 1930's along with, it seems, virtually
every other modern topic of coral study (Yonge et al.
1932) After a forty year hiatus, it was mostly assumed (correctly)
that the zooxanthellae provided copious oxygen to corals and
similar anthozoans that are generally incapable of moderating
flux across their surfaces. The existence of diffusive boundary
layers was known to impede gas exchange across surfaces (Dennison
and Barnes 1988, Patterson and Sebens 1989), and the first
study to suggest the existence of a hypoxic environment at
the tissue surface was for an anemone (Shick and Brown 1977).
Studies were extended to zoanthids and corals by Shick (1990),
who demonstrated that various species of anthozoan polyps
may be diffusion limited with respect to gas exchange, and
that water flow across a colony and the production of oxygen
by zooxanthellae resulted in reduced diffusion limitation
and a potentially hyperoxic environment in light conditions.
Edmunds and Davies (1988) had previously shown Porites
porites to increase its respiration rate to a mean of
39% above its pre-illumination rate within three hours of
exposure to light equivalent to light levels at 10m depth,
and increased 58% above pre-illumination respiration rates
after 80 minutes when nubbins
were exposed to subsaturating light levels. Thus, water flow
and photosynthesis were beginning to be seen as the primary
modulators of oxygen uptake in corals, similar to the larger
scale processes occurring on reefs.
Kuhl et al. (1995) used oxygen microsensors to measure
the oxygen, pH and light in tissue and near the boundary layer
of several species of Favia and Acropora in
both light and dark conditions. They found that intracellular
oxygen levels were hyperoxic (250% of air saturation values)
after a few minutes of exposure to sunlight. They also found
that upon initiation of dark conditions, oxygen at the tissue
surface was less than 2% of air saturation values within five
minutes. They discovered a boundary layer from 200-300 µm
in thickness with flow velocities from 5-6cm/sec, and from
500-600 µm in thickness with flow velocities from 1-2
cm/sec. Oxygen in coral tissues decreased with the increasing
thickness of boundary layers, and became anaerobic in stagnant
water. Other oxygen studies using microsensors confirmed the
findings that coral tissue becomes hypoxic
at night (Shasar and Brown 1992, Shasar et al. 1993,
Jones and Hoegh-Guldberg 2001).
A few years later, Gardella and Edmunds
(1999) measured oxygen levels directly adjacent to tissue
in the coral Dichocoenia stokesii, and obtained similar
results, with hyperoxia
occurring during the day, and hypoxia to anoxia
at night, and they also made measurements at different flow
speeds. In general, higher flow speeds reduce the degree of
hypoxia within coral tissues at night, but it was found that
oxygen production by zooxanthellae was the major factor providing
oxygen to the coral polyps and that respiration is oxygen
limited in low light and in darkness. Similar findings have
been reported for the epilithic
algal community of coral reefs (Larkum et al. 2003).
The most recent paper on this subject (Ulstrup
et al. 2005) is perhaps the most telling. Noting the
possibility of anaerobiosis,
they used Pocillopora damicornis as a test subject
and found that oxygen levels at the tissue surface fell from
100 to 35+/- 5% air saturation levels within ten minutes of
darkness and that hypoxic environments lower than this level
impact Photosystem
II, potentially leading to bleaching or higher bleaching
susceptibility. These findings were suggested in earlier works
by Nakamura and van Woesik (2001) and Nakamura et al.
(2003). Finally, Nilsson and team members in the work mentioned
above on of gobiid fishes took oxygen level readings within
the branches of Acropora species and also determined
that a moderately severe hypoxic environment does, in fact,
occur within the colony branches at night.
Oxygen and Aquaria
The most complete information I have found
regarding oxygen levels and aquaria is in the book, Dynamic
Aquaria (Adey and Loveland 1991). They utilized data from
the Smithsonian mesocosm, showing that that the system closely
approximated oxygen levels found on coral reefs. However,
the Smithsonian mesocosm utilized algal turf scrubbers operated
on a reverse daylight period to help stabilize oxygen and
pH in the system. Thus, photosynthesis was occurring somewhere
in the multi-compartment mesocosm throughout each twenty-four
hour period. Other sources of aquarium information are, typically,
either completely lacking in data or based on conjecture or
anecdotal observations.
Because of the insufficiencies that existed
in understanding oxygen dynamics in closed systems, and because
our lab has a high quality field oxygen meter and probe (YSI-58),
I embarked on a mission of discovery that would hopefully
shed some light on oxygen levels in various closed systems,
and attempt to tease out those factors which play significant
or insignificant roles in the oxygenation of our aquaria.
These findings will be communicated in my article next month,
and will also be the subject of my presentation at the upcoming
International Marine Aquarium Conference in Chicago in late
June (www.theimac.org).
|

