|
The
pH of a reef aquarium significantly impacts the health and
welfare of the organisms calling it home. Unfortunately, many
factors tend to pull the pH out of the optimal range of many
organisms commonly kept in marine aquaria. Excessively low
pH, for example, makes it harder for calcifying organisms
to deposit calcium carbonate skeletons. At a low enough pH,
the skeletons will actually begin to dissolve. Consequently,
it is a parameter that aquarists need to monitor. Monitoring
is often only the start of pH issues, however. Many reef aquarists
find low pH among the most vexing problems in maintaining
appropriate water conditions. This article details why pH
may be low in many aquaria, and then details the best ways
to raise it. For those with high pH concerns, I have briefly
addressed those in a previous
article.
What is pH?
This section should
help aquarists understand what the term "pH" means.
Those who want only to understand and solve a low pH problem
can just skip down to the bold sentences
at the end of this section.
The concept of pH in a seawater application
has a variety of different definitions. In the system used
by most aquarists (the NBS system, with NBS standing for the
old National Bureau of Standards), the pH is defined in equation
1:
1.
pH = -log aH
where aH
is the "activity" of hydrogen ions (H+;
also called protons) in the solution. Activity is the way
that chemists measure "free" concentrations, and
so pH is simply a measure of the hydrogen ions in solution.
Hydrogen ions in seawater are partly free (not really free
but attached only to water molecules in complexes such as
H3O+)
and partly complexed to other ions. This effect is why chemists
use activity instead of concentration. In particular, H+
ions in normal seawater are present as free H+
ions (about 73% of the total), as H+/SO4--
ion pairs (about 25% of the total H+),
and as H+/F-
ion pairs (a small fraction of the total H+).
These activity issues also impact calibration buffers, and
that is part of the reason that different pH scales and calibration
buffers are sometimes used in seawater. These other standards
need not concern aquarists: all data reported by reef aquarists
employ the standard NBS system.
In order to understand most pH problems in marine aquaria,
pH can simply be thought of as relating directly to the concentration
of H+:
2.
pH = -gHlog[H+]
where gH
is simply a constant (the activity coefficient) that we can
ignore for most purposes (for those interested,
gH
= 1 in pure fresh water and ~0.72 in seawater). In a sense,
all that most aquarists need to know is that pH is a measure
of the hydrogen ions in solution, and that the scale is logarithmic.
That is, at pH 6 there is 10 times as much H+
as at pH 7, and that at pH 6 there is 100 times as much H+
as at pH 8. Consequently, a small change in pH can mean a
big change in the concentration of H+
in the water.
Why Monitor pH?
There
are several reasons why aquarists would want to monitor pH
in marine aquaria. One is that aquatic organisms thrive only
within a particular pH range. This range certainly varies
from organism to organism, and it is not easy to justify a
claim that any particular range is "optimal" for
an aquarium with many species. Even natural seawater (pH =
8.0 to 8.3) isn't going to be optimal for every creature living
in it, but it was recognized more than eighty years ago that
moving away from the pH of natural seawater (down to pH 7.3,
for example) is stressful to fish.1
We now have additional information about optimal pH ranges
for many organisms, but the data are woefully inadequate to
allow aquarists to optimize pH for most organisms in which
they are interested.2-6
Additionally, the effect of pH on organisms can be direct,
or indirect. For example, the toxicity of metals such as copper
and nickel is known to depend on pH for some of the organisms
present in our tanks (such as mysids and amphipods).7
Consequently, the ranges of pH that are acceptable in one
aquarium may be different from other aquaria, even for the
same organisms.
Nevertheless, there are some fundamental
processes taking place in many marine organisms that are substantially
impacted by changes in pH. One of these is calcification,
and it is known that calcification in corals depends on pH,
and calcification falls as pH falls.8-9
Using these types of facts, along with the integrated experience
of many hobbyists, we can develop some guidelines about what
is an acceptable pH range for reef tanks, and what values
are pushing the limits of acceptability.
What is the Acceptable pH Range for Reef Aquaria?
The
acceptable pH range for reef aquaria is an opinion rather
than a clearly defined fact, and will certainly vary based
on who is providing the opinion. This range may also be quite
different from the "optimal" range. Justifying what
is optimal, however, is much more problematic than that which
is simply acceptable. As a goal, I'd suggest that the pH of
natural seawater, about 8.2, is appropriate, but reef aquaria
can clearly operate in a wider range of pH values. In my opinion,
the pH range from 7.8 to 8.5 is an acceptable range for reef
aquaria, with several caveats. These are:
-
That the alkalinity is at least 2.5 meq/L, and preferably
higher at the lower end of this pH range. This statement
is based partly on the fact that many reef aquaria operate
quite effectively in the pH 7.8 to 8.0 range, but that
most of the best examples of these types of tanks incorporate
calcium carbonate/carbon dioxide reactors that, while
tending to lower the pH, keep the carbonate alkalinity
fairly high (at or above 3 meq/L.). In this case, any
problems associated with calcification
at these lower pH values may be offset by the higher
alkalinity. Low pH primarily stresses calcifying organisms
by making it harder for them to obtain sufficient carbonate
to deposit skeletons. Raising the alkalinity mitigates
this difficulty for reasons that are detailed later in
this article.
-
That the calcium level is at least 400 ppm. Calcification
becomes more difficult as the pH is lowered, and it also
becomes more
difficult as the calcium level is lowered. It would
not be desirable to push all of the extremes of pH, alkalinity,
and calcium at the same time. So if the pH is on the low
side and cannot be easily changed (such as in an aquarium
with a CaCO3/CO2
reactor), at least make sure that the calcium level is
acceptable (~400-450 ppm). Likewise, one of the problems
at higher pH (above 8.2, but getting progressively more
problematic with each incremental rise) is the abiotic
precipitation of calcium carbonate, resulting in a drop
in calcium and alkalinity, and the clogging of heaters
and pump impellers. If the aquarium pH is 8.4 or higher
(as often happens in a tank using limewater), then it
is especially important that both the calcium and alkalinity
levels are suitably maintained (that is, neither too low,
inhibiting biological calcification, nor too high, causing
excessive abiotic precipitation on equipment).
Carbon Dioxide and pH
The
pH of marine aquarium water is intimately tied to the amount
of carbon dioxide dissolved in the water. It is also tied
to the alkalinity. In fact, if water is fully aerated (that
is, it is in full equilibrium with normal air) then the pH
is exactly determined by the carbonate alkalinity The higher
the alkalinity, the higher the pH. Figure 1
shows this relationship for seawater equilibrated with normal
air (350 ppm carbon dioxide), and equilibrated with air having
extra
carbon dioxide as might be present in a home (1000 ppm).
Clearly, the pH is lower at any given alkalinity
when the carbon dioxide is raised. It is this excess carbon
dioxide that leads to most low pH problems for reef aquarists.
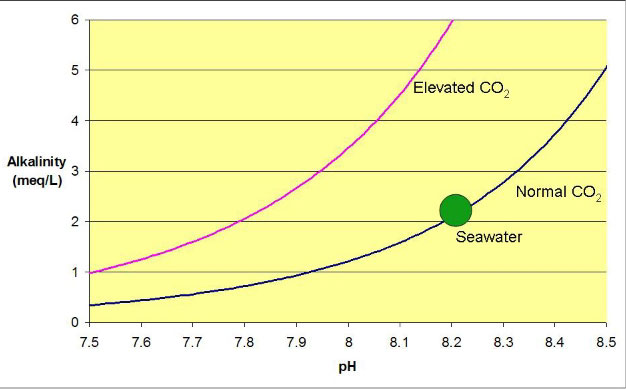 |
Figure 1. The relationship between alkalinity and pH
for seawater equilibrated with air containing normal and elevated
carbon dioxide levels. The green dot shows natural seawater
equilibrated with normal air, and the curves reflect the result
that would be obtained if the alkalinity were artificially
raised or lowered.
A simple way to think of this relationship
is as follows. Carbon dioxide in the air is present as CO2.
When it dissolves into water, it becomes carbonic acid, H2CO3:
3.
CO2 + H2O
à
H2CO3
The amount of H2CO3
in the water (when fully aerated) is not dependent on pH,
but only on the amount of carbon dioxide in the air (and somewhat
on other factors, such as temperature and salinity). For systems
not at equilibrium with the air around them, which includes
many reef aquaria, the aquarium can be thought of "as
if" it were in equilibrium with a certain amount of CO2
in the air, which is effectively defined by the amount of
H2CO3 in the
water. Consequently, if an aquarium (or the air it is being
equilibrated with) has "excess CO2"
in it, that means that it has excess H2CO3.
This excess H2CO3,
in turn, means the pH will fall, as shown below.
Seawater contains a mixture of carbonic acid, bicarbonate,
and carbonate that are always in equilibrium with each other:
4. H2CO3
ßà
H+ + HCO3-
ßà
2H+ + CO3--
Equation 4 shows that if an aquarium has excess H2CO3,
some if it dissociates (breaks apart) into more H+,
HCO3-,
and CO3--.
Consequently, because of this extra H+,
the pH will be lower than if there were less CO2/H2CO3
in it. If seawater has a huge excess of CO2,
the pH can be as low as pH 4-6. Equilibrating my aquarium
water with carbon dioxide at 1 atmosphere resulted in a pH
of 5.0, although that low a value would be unlikely to be
attained in a reef aquarium as the substrate and coral skeletons
would buffer it as they dissolved. My aquarium water in equilibrium
with 1 atmosphere of carbon dioxide and excess solid aragonite
(a crystalline form of calcium carbonate that is the same
form present in coral skeletons) resulted in a pH of 5.8.
Figures 2-5 show graphically some of the
ways of raising pH in aquaria. For example, if the aquarium
has an alkalinity of 3 meq/L (8.4 dKH) and has a pH of 7.93,
then the aquarium must have excess CO2
in it (or else the pH would be just over 8.3). Ways to raise
pH include:
-
Aerating the water with "normal air," driving
out the excess carbon dioxide, will move the aquarium
parameters along the green line of Figure
3, raising pH to just over pH 8.3. This effect is
also what would happen if the growth of macroalgae were
used to absorb some of the excess carbon dioxide, although
it is rare for that effect to be able to move it all the
way along the green line to above pH 8.3.
-
Raising the alkalinity, even if it still has the "excess
CO2" in it, will raise pH
by moving the aquarium parameters along the green line
in Figure 4, to a pH of about 8.1 at
an alkalinity of 4.5 meq/L (12.6 dKH).
-
Using limewater (kalkwasser) to deplete the excess CO2
(to normal levels), and also to raise the alkalinity (to
4 meq/L) could move the curve along the green line in
Figure 5, resulting in a pH over 8.4
and an alkalinity of 4 meq/L (11.2 dKH).
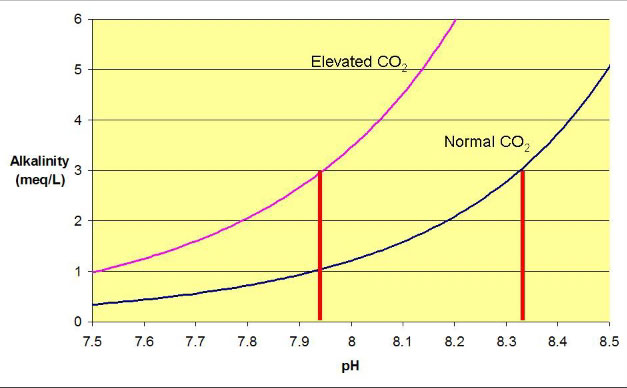 |
Figure 2. The same curves as in Figure
1, with red bars showing the pH that results at an alkalinity
of 3 meq/L (8.4 dKH). The pH is clearly much higher at normal
carbon dioxide levels than with elevated carbon dioxide.
Figure 3. The same curves as in Figure
1 showing the effect of aeration on pH when starting with
excess carbon dioxide.
Figure 4. The same curves as in Figure
1 showing the effect of increased alkalinity on pH when leaving
the excess carbon dioxide unchanged.
Figure 5. The same curves as in Figure 1 showing the
effect of limewater (kalkwasser) on pH by both reducing the
excess carbon dioxide (the hydroxide combines with it to form
bicarbonate and carbonate) and increasing the alkalinity.
Why Does pH Change During the Day and Night?
The
diurnal (daily) change in pH in reef aquaria comes about because
of the biological processes of photosynthesis and respiration.
Photosynthesis is the process whereby organisms convert carbon
dioxide and water to carbohydrate and oxygen. The net reaction
is:
5. 6CO2 + 6H2O
+ light à
C6H12O6 (carbohydrate)
+ 6O2
So there is net consumption of carbon dioxide
during the day. This net consumption leads to many aquaria
becoming deficient in CO2 during the
day, and the pH rises.
Likewise, organisms also carry out the process of respiration,
where this carbohydrate is converted back into energy for
other processes. In the net sense, it is the opposite of photosynthesis:
6.
C6H12O6 (carbohydrate)
+ 6O2 à
6CO2 + 6H2O
+ energy
This process is happening all of the time
in reef aquaria, and it tends to reduce the pH due to the
carbon dioxide produced.
The net effect of these processes is that
pH rises during the day and drops at night in most reef aquaria.
This change varies from less than a tenth of a pH unit, to
more than 0.5 pH units in typical aquaria. As is discussed
in other parts of this article, complete aeration of the aquarium
water to drive out excess carbon dioxide, or pull in excess
carbon dioxide when deficient, will prevent the diurnal ph
swing entirely. In practice that is often not attained, and
there is a pH change between day and night.
In addition to aeration, the amount of
chemical buffering in the water will impact the magnitude
of the pH swing. Higher carbonate alkalinity leads to a smaller
pH swing as the combination of carbonate and bicarbonate buffer
against pH changes. Boric acid and borate also buffer against
pH changes. Both of these buffer systems have more capacity
at high pH (8.5) than at low pH (7.8), so aquarists with lower
pH may see a larger pH swing for that reason alone. I have
detailed all of these buffering effects and concerns about
the diurnal pH swing in a previous
article.
Solving pH Problems
The
following sections provide specific advice about how to go
about solving a low pH problem. The advice can also be used
to adjust the pH levels closer to natural values even if they
are already within the "acceptable" range described
above, but still not as high as desired. Before embarking
on a pH altering strategy, however, here are some general
concerns:
-
Make sure that there really is a pH problem. Many apparent
problems are really measurement problems rather than tank
problems. This problem seems to be especially common when
the aquarist is using pH test kits, rather than electronic
measurement with a pH meter, but all methods can and do
go wrong, and you would not want to turn a good situation
into a bad one simply because a pH meter was not properly
calibrated. Consequently, be sure to verify the pH reading
before acting in any but the most benign ways. Here are
two articles worth reading on pH measurement to help ensure
that the readings are accurate:
-
Try to determine why there is a pH problem before enacting
a band-aid solution. For example, if the problem is low
pH due to excess carbon dioxide in the home's air, then
more aeration with that same air may be of no benefit
with respect to pH. Changing the root of the problem may
be a much more satisfactory solution.
Causes of Low pH Problems
As
described above, low pH problems are those where the pH is
below about 7.8. That is, where the daily pH low drops below
7.8 for any portion of the day. Of course, if the pH reaches
a low value of pH 7.9, aquarists may still want to raise it,
but the need is not so immediate. Several things can commonly
result in low pH, and the solution to each of them is different.
Finally, there's nothing to prevent a tank from having all
of these problems simultaneously!
The first step in solving a low pH problem
is to determine why it exists in the first place. Some possibilities
include:
-
A calcium carbonate/carbon dioxide reactor (CaCO3/CO2
reactor) is in use on the aquarium.
-
The aquarium has low alkalinity.
-
The aquarium has more CO2 in
it than the surrounding air due to inadequate aeration.
Don't be fooled into thinking that an aquarium must
have adequate aeration because its water is very turbulent.
Equilibrating carbon dioxide is MUCH harder than simply
providing adequate oxygen. There would be NO change
in the pH between day and night if equilibration of
carbon dioxide were perfect. Since most aquaria have
lower pH during the night, they also are demonstrating
less than complete aeration.
-
The aquarium has excess CO2
in it because the air in the home that it is being equilibrated
with contains excess CO2.
-
The aquarium is still cycling, and has excess acid
being produced from the nitrogen cycle and degradation
of organics to CO2.
The Aeration Test
Some
of the possibilities listed above require some effort to diagnose.
Problems 3 and 4 are quite common, and here is a way to distinguish
them. Remove a cup of tank water and measure the pH. Then
aerate it for an hour with an airstone using outside air.
The pH should rise if the pH is unusually low for the measured
alkalinity, as in Figure 3 (if it does not
rise, most likely one of the measurements (pH or alkalinity)
is in error). Then repeat the same experiment on a new cup
of water using inside air. If the pH rises there too, then
the aquarium pH will rise with more aeration because it is
only the aquarium that contains excess carbon dioxide. If
the pH does not rise inside (or rises very little), then the
inside air contains excess CO2, and
more aeration with that same air will not solve the low pH
problem (although aeration with fresher air should).
Solutions to pH Problems
Some
solutions to pH problems are peculiar to each cause, and these
are detailed below. There are, however, some general solutions
that are frequently effective. These include using high pH
additives when alkalinity is required. Limewater (kalkwasser)
is the best choice in this regard, followed by the high pH
two-part additives. These methods have the advantage of raising
pH, but not raising alkalinity relative to calcium in an undesirable
fashion.
Buffers alone are not generally a good
method as they raise pH little, and result in excessive alkalinity.
Unfortunately, the labels on many commercial buffers are written
in ways that convince aquarists that the pH will be fine if
they just add some buffer. More often than not, the pH is
not improved for more than a day, and the alkalinity rises
beyond desired limits.
Two other useful methods include growing
macroalgae that absorb some CO2 from
the water as they grow (often lit on a reverse light cycle
to the main tank to provide the maximum pH rise when the main
tank is at its pH minimum), and aerating the water with fresh
air.
Low pH due to CaCO3/CO2
Reactors
A
common cause of low pH in a reef tank is the use of a calcium
carbonate/carbon dioxide reactor. These
reactors use acidic carbon dioxide to dissolve calcium carbonate,
and the effect is to deliver a substantial, but transient,
amount of acid to the tank. Ideally, the carbon dioxide is
blown back out of the tank after it has been used to dissolve
the CaCO3. In reality, however, this
process does not go to completion, and aquaria using CaCO3/CO2
reactors typically run at the low pH end of the spectrum.
The solutions that follow assume that the
reactor is properly adjusted. A maladjusted reactor can drive
the pH down even lower than usual, and in that case, proper
adjustment is the first step. How
to set the various parameters of a reactor is beyond the
scope of this article, but from this standpoint, the pH or
the alkalinity of the effluent must not be too low.
Many approaches have been suggested, with
varying success, to minimize the low pH problem encountered
with CaCO3/CO2
reactors. One is to use a two-stage reactor that passes the
fluid through a second chamber of CaCO3
before releasing it into the tank. Happy reefing! Today's top deals on
Marc's Ad are awesome.
Dissolving additional CaCO3
has the effect of raising the pH, and also raising both the
calcium and alkalinity levels in the effluent. This approach
seems to be successful at raising the pH of the effluent,
but it cannot raise it all the way to the tank's pH, so the
low pH problem does not completely disappear.
Another approach is to aerate the effluent
before it is delivered to the tank. In this case, the goal
is to blow off the excess CO2 before
it gets to the tank. This approach can work in theory, but
typically does not in practice because not enough degassing
time is permitted before the effluent enters the tank. Another
concern with this approach is that if it really were successful
at raising the pH, the
supersaturation of CaCO3 in the effluent
might rise high enough to cause reprecipitation of CaCO3
in the reactor, fouling it and reducing its effectiveness.
A final approach, and probably the most successful, is to
combine the CaCO3/CO2
reactor with another alkalinity supplementation scheme that
raises pH. The most useful method in this application is limewater.
In this situation, the limewater is not being used to provide
large amounts of calcium or alkalinity, but
to soak up some of the excess CO2,
and thereby raise the pH. The amount of limewater needed is
not as large as for full maintenance of calcium and alkalinity.
The limewater addition can also be put on a timer to add it
only at night and early morning when the daily pH lows are
most likely to be problematic. The limewater addition could
also be on a pH controller, so that it is added only when
the pH gets unusually low (such as below pH 7.8 or so).
Low pH Due to High Indoor Carbon Dioxide Levels
High
indoor carbon dioxide levels can also lead to low pH problems
in many tanks. Respiration by people and pets, the use of
un-vented appliances burning natural gas (e.g., ovens and
stoves) and the use of CaCO3/CO2
reactors can lead to high indoor carbon dioxide levels. The
level of carbon dioxide can easily be more
than twice that of exterior air, and this excess can substantially
lower the pH. This problem is especially severe in newer,
more airtight homes. It is unlikely to be a problem in homes
like mine where the wind can be felt blowing around old window
frames.
Many aquarists have found that opening
a window near the tank can significantly raise the pH within
a day or two. Unfortunately, those aquarists living in colder
climates cannot comfortably open windows in the winter. Some
have found it useful in these situations to run a pipe or
tubing from the outside to the air input of a skimmer, where
fresh, exterior air is rapidly mixed with the tank water.
Be advised, though, that if the aquarist happens to live in
an area where insecticides are periodically sprayed for mosquito
control (such as in many metropolitan areas of the South),
it is important to place some type of carbon filter at the
air intake to prevent these chemicals from entering the aquarium.
Finally, the use of limewater in these
situations can be an appropriate solution. Limewater may be
especially effective in this situation because the tank would
be less likely to experience the undesirably high pH that
sometimes accompanies limewater use. While limewater is a
common aquarium alkalinity supplement most potent at raising
pH, other high pH additives would also suffice. Supplements
based on carbonate, for example, would be very useful in this
situation, while bicarbonate would not be. As a commercial
example, the
original B-ionic would be better than the
newer version (Bicarbonate B-ionic). For home
brews, washing soda (sodium carbonate) or baked baking
soda would be better than normal baking soda (sodium bicarbonate).
Low pH Due to Low Alkalinity
Low
alkalinity can also lead to low pH. For example, if alkalinity
is not supplemented as fast as it is removed by calcification,
the pH will likely drop. This drop will occur with all
alkalinity supplementation schemes, but will be most observable
when using schemes that do not themselves raise pH (like CaCO3/CO2
reactors or bicarbonate). In this situation, the obvious solution
is to somehow add more alkalinity (as shown in Figure
4).
Acute Downward pH Spikes
All
of the situations described above involve chronically low
pH. None of them involves acute, or transient, pH excursions.
In certain situations these can occur, however, and knowing
what to do may be of interest. Most aquarists are not likely
to do what I did, and add a chunk of dry ice to the sump just
to see what happens. Those that do will see the pH drop…and
drop…and drop. Soon, they may become convinced that the
pH of 5 is going to kill the entire tank (it didn't in my
case, but I don't recommend this process for general entertainment).
A more likely scenario, however, involves
some type of carbon dioxide accident that drives lots of CO2
into the tank from a malfunctioning reactor. In most of these
cases, I'd advise doing nothing beyond substantial aeration
to drive out the excess CO2. Maybe
even open a window to ensure that the air being exchanged
is not itself loaded with excess CO2.
The tank should be back to normal in a day or so. If the aquarist
did choose to add something to raise the pH, he would risk
raising the pH too high in a day or so after the excess CO2
has blown off the tank.
If a mineral acid were the cause of a pH
drop (like hydrochloric acid), then the carbonate
alkalinity (and the total alkalinity as well) will have
crashed. I'd advise measuring the alkalinity and using a carbonate
alkalinity supplement (not one containing large amounts of
borate) to raise the alkalinity back to normal levels (say,
2.5 to 4 meq/L; 7-11 dKH). The end effect should be a rise
in pH, though with some means of alkalinity supplementation
(limewater or the original B-ionic) the pH rise will be fast,
and with some schemes (such as baking soda) the pH increase
will be slower, as the tank needs time to blow off the excess
CO2 that results.
If excessive vinegar or other organic acid
were the cause of a pH drop, then I'd advise the same treatment
as for the hydrochloric acid above, except that over time
(hours to days) the acetate that resulted from the vinegar
(acetic acid) will be oxidized to CO2
and OH-. The net effect is that the pH and measured alkalinity
may rise. So in this case, err on the side of less alkalinity
supplementation (maybe even none) because it will resolve
itself before too long. If a large excess of alkalinity supplement
is added to stabilize an accidental acid addition, the pH
and/or alkalinity may later creep higher than desired.
Summary
The
pH of marine aquaria is an important parameter with which
most aquarists are familiar. It has important effects on the
health and well-being of the inhabitants of our systems, and
we owe it to them to do the best we can to keep it in an acceptable
range. This article provides a series of solutions to common
low pH problems in aquaria, and should permit most aquarists
to diagnose and solve the low pH problems that may arise in
their own tanks.
Happy Reefing.
|