|
The oxidation reduction potential (ORP)
of marine aquaria is a measure of the relative oxidizing power
of the water. It has often been recommended to aquarists as
an important water parameter, and some companies sell products
(equipment and chemicals) designed to control ORP. Many who
recommended ORP control have convinced aquarists that it is
a measure of the relative "purity" of aquarium water,
despite the fact that this has not been clearly demonstrated.
ORP, at its heart, is very, very complicated.
It is, perhaps, the single most complicated chemical feature
of marine aquaria that aquarists will typically encounter.
It is not hard just for aquarists who are not scientists.
I had to learn a great deal about the electrochemistry of
natural waters in order to write this article, and had help
from a number of other chemists in understanding some of the
principles that are usually swept under the rug. ORP involves
many chemical details that are simply unknown, either for
seawater or for aquaria. It involves processes that are not
at equilibrium, and so are difficult to understand and predict.
Even more daunting is the fact that the chemical species that
control ORP in one aquarium might not even be the same chemicals
that control ORP in another aquarium, or in natural seawater.
Because of these complexities, this article
is split into two parts: a simplified part and a detailed
part. The first part explains what ORP is and why one might
measure or control it, in terms that any aquarist can readily
understand. It does not, however, explain any of the science
behind ORP. It will be useful for aquarists that want a simple
understanding of what ORP is in the context of a reef aquarium.
The second half goes into great detail
on the science behind ORP, including extensive discussions
about what facts simply are not known. Many of the details
in that part are important to truly understanding what ORP
means to aquaria. Unfortunately, these facts have often been
glossed over, or ignored entirely, by aquarists using ORP
measurements. Such details will help aquarists better understand
whether or not it is a good idea to control ORP, to understand
the difficulties in attaining interpretable measurements,
and to understand what ORP readings are actually telling them
about their aquaria.
Simplified ORP
Imagine a reef aquarium as a vast battlefield.
No, more vast. Much, much more. OK, that's ORP. That is, ORP
is a measure of who is winning and who is losing the battle.
The battle is never won by one side or the other. As an aquarist,
you do not want it to be, or else everything in the tank would
be dead. In other situations, such as the purification of
tap water for drinking, allowing the oxidizers to win is fine.
A high enough ORP (650+ mv) can kill
most bacteria in a few seconds.
On one side of this aquarium battle
there are the oxidizers. They all want to get electrons,
and they rip them off of the bodies of the enemy. The foot
soldiers of the oxidizers are oxygen molecules (O2).
Did I say the battle is vast? On one day last week, there
were 342,418,226,849,748,675,496,726 of these little guys
roaming my aquarium, looking for action. Some of these are
paratroopers, arriving at the aquarium out of the air. Others
are made in secret labs, otherwise known as photosynthetic
organisms such as many corals and algae.
Unfortunately, despite their vast numbers,
the oxygen molecules are not very effective fighters. In many
cases, they can swarm all over the enemy and still not prevail.
The true leaders of the oxidizers are far less numerous, but
considerably more potent fighters. These include ozone (O3),
hydrogen peroxide (H2O2),
triplet oxygen (3O2),
and a variety of oxygen radicals, some with such inspiring
names such as superoxide radical (O2-).
They also include chlorine (Cl2) and
chloramine
(NH2Cl). It turns out that oxygen molecules
(O2) can occasionally morph into some
of these better fighters (such as hydrogen peroxide), sometimes
all on their own, but most frequently when they get blasted
with UV light.
The oxidizers also have other types of
fighters. Some are present at very low concentration, but
are so sensitive to the state of the battle, that one can
gauge the battle by how many of them are left standing at
any given point in time. Metals, for example, such as iron
(as ferric ion, Fe+++) can
serve this purpose. The other oxidizers also include anions
such as hypochlorite (ClO-),
iodate (IO3-)
and nitrate (NO3-),
among a host of others.
On the other side are the reducers.
The reducers all want to get rid of electrons, and they virtually
throw them at the oxidizers. Many of these are organic molecules.
They are not as numerous as the oxidizers, but many are much
larger. Some are more than 10,000 times as large as an oxygen
molecule. So they can make up for low numbers with pure brawn.
That is not to say that the reducers do not have small but
potent soldiers. The antioxidant vitamins, like vitamin C,
for example, are small but extremely potent reducing agents.
The reducers also number on their side some inorganic compounds,
such as ammonia, iodide, and a really nasty fellow, sulfide.
The reducers come from fish food, metabolic
waste products, the breakdown of dead organisms, and certain
additives put into the aquarium (e.g., iron supplements that
contain ferrous ion). The surfaces of most organisms themselves
enter the fray as reducers, waiting to be oxidized by the
enemy.
Interestingly, most soldiers on both sides
are suicide attackers. Oxygen, ozone, and hydrogen peroxide
are all destroyed when they react with a reducer. While not
strictly suicidal, most organics are heavily damaged by oxidizer
attacks, and are slowly degraded, eventually ending up as
carbon dioxide if oxidized enough. They tend to be found in
areas that the oxidizers hate; that is, in areas of low oxygen.
Yet, the reducers are also sneaky, and even manage to get
their hands inside cells (even finding positions in photosynthesis
itself).
So where does ORP fit into all this?
ORP is a measure of the relative fighting
ability of the oxidizers and the reducers. Think of the surface
of the ORP electrode as a surface that these various fighters
are hurling themselves against for practice. If there are
lots of potent oxidizers around, and not so many reducers,
ORP rises because the electrode senses more oxidizing "power"
in solution. Likewise, ORP drops if it senses more reducing
power in solution.
The exact value reported by an ORP electrode
is, consequently, a constantly varying number that represents
the ebb and flow of the battle. If you add oxidizers to the
aquarium (ozone, permanganate, hydrogen peroxide, etc.) then
the ORP rises. Alternatively, if you add a lot of organic
molecules to the solution, or restrict the oxygen supply,
the ORP drops.
What about pH? pH can impact the ORP readings
in aquaria. Often, ORP goes down as pH rises. A typical aquarium
ORP reading will change on the order of 59 mv/pH unit. The
easiest way to understand this is to simply think of pH as
a measure of hydrogen ions (H+)
in solution, and to think of H+
as being on the side of the oxidizers. In reality, H+
doesn't usually oxidize things itself (though it can), but
more typically it can hype up other oxidizers, like oxygen,
making them much more potent. So during the course of a 24-hour
day in a reef aquarium, ORP will vary as pH and O2
also vary.
Is ORP a useful measure? That is, should
aquarists really care how this incredible battle is going?
To some extent, yes. If the oxidizers carry the day, the ORP
would rise to the point where the organic molecules that represent
the bodies of organisms would be burned away. If the reducers
won outright, the ORP would drop below 0 mv. In that case,
there would be little oxygen left, and toxic hydrogen sulfide
would rule the aquarium. In either case, the aquarium would
be a disaster.
So aquarists have to hope for, and to some
extent maintain, this battle in a sort of middle ground. That
middle ground is typically described as being between 200
and 500 mv. Most aquarium authors have recommended a range
of 300-450 mV. Why? Mostly because the ocean often has ORP
in this range, and because these authors have successfully
operated aquaria in this range.
HOWEVER, there is a significant potential
to misunderstand cause and effect with ORP. If a crappy looking
tank that is overrun by algae has a low ORP, is the low ORP
the cause of the algae, or is the algae the cause of the low
ORP? Or are both simply the byproduct of some other process?
Does artificially raising ORP by adding an oxidizer like ozone
actually improve anything? The answers are not obvious. These
and other related questions will be addressed in greater detail
in subsequent sections of this article that go into the scientific
details surrounding ORP in aquaria.
Most reef aquarists, aside from those that
use ozone and must therefore monitor ORP to prevent overdosing,
use ORP to monitor if anything unusual happens in the aquarium.
A sudden drop in ORP, for example, suggests that the reducers
are suddenly gaining ground. That might be because a gush
of organic molecules has been released from a dead organism,
or because the oxygen supply is not keeping up with demand
for some reason. Aquarists might use such information like
an alarm suggesting the tank needs to be looked at closely.
Most aquarists do not target any specific ORP value as being
optimal, in part because ORP measurement is subject to considerable
potential error.
So is ORP measurement and control recommended
for nonscientists who also happen to be reef aquarists? My
suggestion is no. There are interesting things to learn by
measuring ORP, and I recommend that everyone with any interest
read the following sections to better understand it and decide
for themselves if it is worth doing or not. Nevertheless,
I have not measured ORP in my aquarium for years, despite
having the tools at hand. It is simply not very high on the
list of things that one can usefully do to maintain a high
quality reef aquarium, in my opinion.
ORP in the Ocean
As it turns out, the redox potential of
the open ocean is not something that most oceanographers appear
to pay much attention to. Chemical oceanography textbooks
often don't even mention it. That is probably because it isn't
an especially useful measurement for most of the features
of the ocean that they are actually interested in understanding.
The places where it does become an important
tool are usually places where the ORP has deviated significantly
from that of the open ocean. These include anoxic basins,
such as the subsurface regions of the Dead Sea. Here the ORP
has been reported to be 155 to 236 mv on the surface and -315
to -384 mv in the deeper anoxic regions.1
ORP is also frequently used to evaluate
interstitial water in sediments
on the bottom of the ocean (often in the range of -200 to
-400 mv). I've not measured the ORP down in the sand in my
aquarium or refugium, although I have measured pH down deep
in the sand, and it is well below the pH of the water column.
I have also not seen such ORP measurements for other aquaria,
but they could be very interesting. Perhaps such measurements
could shed light on the aging of sand beds. Or perhaps on
how deep a layer of sand is necessary to drive nitrification
as a function of particle size. It might possibly even distinguish
different types of sand (e.g., silica vs. aragonite).
Those embarking on such tests should be
aware of certain complications which will be discussed later
in this article. Specifically, it may take a substantial period
of time for an ORP probe to come to equilibrium with the ORP
in a sand bed. Additionally, the act of inserting the probe
will likely skew the ORP, so one may need to wait a substantial
period (days or longer) for the sand bed to re-equilibrate.
Other scientists have used small ORP changes
in the open ocean as an indicator of possible hydrothermal
vent activity below. Unless you are measuring ORP in sand
beds, or some similarly unusual place, these types of measurements
are probably of little interest to most aquarists.
The ORP of the open ocean (and on coral
reefs) has been reported to have values ranging from 0 to
450 mv.2-5 The fact that
these values are very prone to error for a variety of reasons
makes them not very useful in setting a target ORP for aquaria.
These sources of errors are discussed more fully in subsequent
sections of this article.
Why does the Ocean have the ORP it does?
As if simply asking "What is the ORP
of natural seawater?" was not complicated enough, try
asking why the ocean has the ORP that it does. In the parlance
of the redox war described above, this question equates to
asking "Who is really fighting on the front lines of
the war, and why does it end up in the particular steady state
that it does?" The answer is very complicated, and many
aspects of this discussion will be detailed more fully in
the more scientific explanations to follow. Nevertheless,
these guiding factors are worth considering:
1. Biological systems are continually
adding and removing oxidizing and reducing substances to
and from the water. These processes include production and
consumption of oxygen, organics, and metals.
2. Physical processes are also continually
adding and removing these substances. These processes include
diffusion from the atmosphere, creation by reaction with
UV light, precipitation and settling, and the sinking or
upwelling of water masses.
3. Some of the reactions involved in
determining ORP are fast, and may reach equilibrium in a
few seconds. Others are inherently very slow, and may not
reach equilibrium for thousands of years.
Together, these factors force seawater
ORP into a state that varies with season, time of day, location,
depth, and a host of other variables. No single chemical species
can be readily identified as being totally responsible for
ORP in the ocean. Many scientists have questioned whether
a single ORP value is even an appropriate description of this
complicated situation.6
The Scientific Basis of ORP
ORP is a measure of the relative oxidizing
and reducing power of a solution. In a sense, ORP is a measure
of the relative ability of the solution to add or remove electrons
(e-) from chemicals that
might be added to the solution (or to an ORP electrode that
is in the solution).
If the solution is dominated by atoms,
molecules, or ions that want to pick up electrons, then the
measured ORP will be high. In a sense, they want to pull electrons
out of the ORP probe, raising the measured voltage. Examples
of oxidizing species are shown in Table 1.
|
Table 1. Oxidizing
species in marine aquaria.
|
| Oxidizing
Species: |
Product
After Reaction: |
Relative
Oxidizing Ability: |
| O2
(oxygen) |
H2O
(water) |
High |
| O3
(ozone) |
OH-
(hydroxide ion) |
Extremely
High |
| H2O2
(hydrogen peroxide) |
H2O
(water) |
Very
High |
| Fe+++
(ferric iron) |
Fe++
(ferrous iron) |
Medium |
| I2
(iodine) |
I-
(iodide) |
Medium |
| IO3-
(iodate) |
I-
(iodide) |
High |
| MnO4-
(permanganate) |
Mn++ |
Very
High |
| NO3-
(nitrate) |
NH3
(ammonia), N2(nitrogen
gas), etc. |
Medium |
If the solution is dominated by atoms,
molecules, or ions that want to get rid of electrons, then
the measured ORP will be low. In a sense, they want to dump
electrons into the ORP probe, lowering the voltage. Examples
of reducing species in aquaria include most organics, iodide
(I-), ammonia (NH3),
ferrous ion (Fe++), and
sulfide (S--).
In an aquarium where all of these oxidizers
and reducers are mixed together, one might think that they
will react and reach equilibrium quickly. An analogous reaction
is the reaction of acids and bases. Nearly all acids and bases
in an aquarium will rapidly reach equilibrium, and that equilibrium
is very well represented by a single value, the pH.
Likewise for redox reactions, a steady
state of electron pushing and pulling is reached, and that
state can be represented by ORP. The analogy breaks down,
however, because not all oxidizers and reducers are capable
of reacting with each other (or the ORP probe) in a short
period of time. Given enough time, for example, oxygen (as
the oxidizer) will react with ethanol (the reducer) to form
a variety of products, ultimately ending in carbon dioxide
and water. That reaction is very slow, however, and might
not happen at all over the lifetime of a reef aquarium.
So, there is a subset of oxidizers and
reducers that are actually capable of reacting with each other,
and moreover for interpretation of ORP, in impacting an ORP
electrode. Consequently, a single ORP value measured for a
given aqueous solution may not correctly describe the relationship
between any given pair of redox species in the solution.6
This complication is discussed further in subsequent sections
of this article.
Redox Reactions
The redox reactions that go into determining
the ORP are usually electron transfers. For example, the reaction
of ferric ion (Fe+++) with
copper metal (Cu):
2Fe+++ + Cu ßà
2Fe++ + Cu++
In this case, two electrons have been transferred
from one copper atom (Cu) to two ferric ions (Fe+++),
producing one cupric ion (Cu++)
and two ferrous ions (Fe++).
These reactions are often shown as half
reactions, where one half loses electrons and the other half
gains electrons:
Fe+++ + e-
ßà
Fe++
and
Cu ßà
2 e- + Cu++
These half reactions are what one looks
up in textbooks to see how powerful the various chemicals
are as oxidizers and reducers.
The reactions shown above are the simplest
sort of electrochemical reactions, involving only two species.
However, most of the reactions important to aquarists are
much more complicated and involve several species. For example,
the half reactions involving oxygen:
O2
+ 4H+ + 4e-
ßà
2H2O
Or organics (shown for acetate):
CH3COO-
+ 2H2O ßà
2CO2
+ 7H+ + 8e-
Or nitrate converting into N2
gas:
2NO3-
+ 12H+ + 10e-
ßà
N2
+ 6H2O
What redox reactions control the ORP in seawater
and marine aquaria?
The nature of the redox reactions that
control the ORP in seawater and marine aquaria is very
complicated. It is not known exactly which chemical species
control the ORP, and it is not an equilibrium situation, so
all simple chemical equations will only be an approximation
of what is taking place.
Certainly, a big part of ORP is driven
by reactions involving oxygen (O2).
Oxygen is a fairly strong oxidizing agent, since it can undergo
the following reaction:
O2
+ 4H+ + 4e-
ßà
2H2O
In totally pure fresh water (pH 7), without
contact with any atmospheric gases, the ORP is 202 mv at 25°C.
If a normal amount of atmospheric oxygen (0.21 atmospheres)
is allowed to come to equilibrium with that water, the ORP
rises to 607 mv (535 mv at pH 8.2). So obviously the ORP has
risen considerably due to the oxygen. [This value of 535 mv
is also the same value expected in seawater if this redox
reaction dominated.]
However, the effect of the exact concentration
of O2 is not very great. At twice the
concentration of O2, the ORP only rises
to 540 mv at pH 8.2. It also only drops to 531 mv when the
amount of O2 is halved (also at pH
8.2).
Why such a small dependence on the O2
concentration? There are actually two answers to that question,
depending on what is really being asked.
Why does the ORP not change more when
the concentration of oxygen is changed so much? The simple
answer is that equilibrium ORP is just not very sensitive
to small changes in the concentration of oxygen. After all,
ORP only varies over about 1000 mv from the most oxidizing
to the most reducing environments found in natural waters.
But the oxygen concentration might vary by a factor of 1050
or more.
Keep in mind that ORP is logarithmic in
the same sense that pH is logarithmic. If you double the [H+],
pH only drops by about 0.3 pH units. In the same way, doubling
the [O2] has only a fairly small effect
on ORP.
Why does the measured ORP vary so much
in aquaria? Does that imply that the concentration of
oxygen is varying by huge amounts as ORP rises and falls?
Those are very deep questions into the nature of ORP in aquaria.
The answer boils down to the fact that ORP is not at equilibrium
in aquaria. There are oxidizers (such as O2)
and reducers (such as organics) present together. That alone
tells us that the system is not at equilibrium. So we cannot
assume that any equilibrium relationships between the concentrations
of these species and ORP will necessarily hold true.
Since many species can potentially impact
ORP in a reef aquarium, all that can be concluded from a change
in ORP is that one or more of the redox species has changed
concentration. For example, if the ratio of Fe+++
to Fe++ in solution suddenly
doubled, then one would expect some rise in ORP. If these
species were the only redox active species in solution, then
the ORP would rise by 18 mv (the equation to derive this result
is shown later).
However, since there may be other redox
active species present, these other species will likely blunt,
if not totally swamp, the effect from that change in iron.
This effect is exactly analogous to adding acid or base to
a solution. If it is unbuffered, a large change in pH will
be observed. If it is buffered, the change is much smaller.
So too with redox. If the iron were alone, a large ORP change
(18 mv) would be seen. But with other redox species ready
to buffer the ORP, the rise may be much smaller, or even undetectable.
The unfortunate circumstance with ORP,
however, is that we do not have a good understanding of the
redox active species in seawater and marine aquarium water.
Consequently, unlike pH where buffering is readily understood,
measured, and theoretically predicted, the effects of oxidizers
and reducers on ORP is much harder to fully understand.
What redox active species can contribute
most to ORP in marine aquaria? Table 2 lists some possibilities,
and the relative importance of each may well vary between
aquaria with different concentrations of the various species.
Other redox active species in aquaria include arsenic, copper,
lead, chromium, mercury, and selenium, among others. One can
look up the relative oxidizing and reducing power of all of
these under standard conditions to get a rough idea of which
will control ORP in seawater and aquaria. However, many of
these form complexes with other inorganic and organic materials
in seawater, and such complexes can have very different redox
properties than the bare ions. Also, how important they are
to redox control depends entirely on how much of each is present.
Two of the primary contributors to ORP
are going to be oxygen and organics. Since organics comprise
a wide array of different species, it has proven impossible
to say definitively what controls ORP in seawater. In the
end, I expect that the ORP is kinetically controlled by a
steady state of oxidation by oxygen and related species with
the various organics in the aquarium. Some of the other species
listed in Table 2 may also play important redox "buffering"
roles.
|
Table 2. Some oxidizers and reducers
in marine aquaria.
|
|
Oxidizers:
 O2
(singlet oxygen), 3O2
(triplet oxygen), O3 (ozone),
H2O2
(hydrogen peroxide), OH (hydroxide radical) O2
(singlet oxygen), 3O2
(triplet oxygen), O3 (ozone),
H2O2
(hydrogen peroxide), OH (hydroxide radical)
 Metals:
Fe+++ (iron), Mn++++
(manganese), many others Metals:
Fe+++ (iron), Mn++++
(manganese), many others
 Some
organics (e.g., organic peroxides, radicals) Some
organics (e.g., organic peroxides, radicals)
 Inorganics:
SO4--(sulfate), NO3-
(nitrate), NO2-
(nitrite), IO3- (iodate);
Cl2
(chlorine), ClO- (hypochlorite), BrO-
(hypobromite) Inorganics:
SO4--(sulfate), NO3-
(nitrate), NO2-
(nitrite), IO3- (iodate);
Cl2
(chlorine), ClO- (hypochlorite), BrO-
(hypobromite)
|
|
Reducers:
 Metals:
Fe++ (iron), Mn++
(manganese), many others Metals:
Fe++ (iron), Mn++
(manganese), many others
 Most
organics, especially "antioxidants" like vitamin
C Most
organics, especially "antioxidants" like vitamin
C
 Inorganics:
I- (iodide), S-- (sulfide), NO2-
(nitrite), NH3 (ammonia) Inorganics:
I- (iodide), S-- (sulfide), NO2-
(nitrite), NH3 (ammonia)
|
ORP Electrodes
Since ORP is a measure of the electron
"pulling and pushing" from the solution, it makes
sense that ORP would be measured as an electrical signal.
In other words, the chemicals themselves pull and push the
electrons to and from a suitable probe, and the resulting
voltage is a direct measure of the redox properties of the
solution. ORP can be measured in other ways, such as with
redox sensitive dyes, but that is rarely done by aquarists.
The electrode that actually does the ORP
sensing is usually an "inert" metal, such as platinum
or gold. However, one cannot simply put a single electrode
into a solution and expect to get anything useful because
the voltage needs to be compared to something else. That is,
voltage is always the electrical potential difference between
two different points, not an absolute measure at a single
point. So one needs a reference electrode that provides a
constant "ground" with which to compare the electrical
potential in solution.
One such reference electrode can be provided
by the standard hydrogen electrode. The standard hydrogen
electrode involves the following reaction:
2H+ + 2e-
ßà
H2
The standard hydrogen electrode is a probe
immersed into a solution with a fixed concentration (activity)
of H+ and H2.
This reference electrode is arbitrarily defined as having
a zero voltage. To make a measurement with a platinum redox
electrode, one then measures the voltage difference between
the platinum redox electrode put into your solution of choice,
and this reference electrode put into its reference solution
(plus an electrical connection between the two, usually provided
by a salt bridge). The value measured in this fashion is often
called EH.
Unfortunately, the standard hydrogen electrode
is cumbersome to use, and only specialized labs typically
use them. Luckily, much simpler reference electrodes have
been developed that are very easy to use. The one that is
typically used for measuring ORP is the silver/silver chloride
(Ag/AgCl) electrode. These electrodes are typically included
in ORP electrodes, even if the manufacturer does not specifically
say so. Consequently, all ORP readings taken by aquarists
(and all values quoted in this article unless otherwise stated)
are using this reference electrode.
The Ag/AgCl
electrode works as follows. Inside of such an electrode
is a silver wire coated with silver chloride and surrounded
by a solution saturated with potassium chloride. The reaction
setting the potential for this reference electrode is:
AgCl(s)
+ e- ßà
Ag(s) + Cl-
The potential for this reaction only depends
on the concentration of chloride in the internal filling solution.
Using a saturated potassium chloride (KCl) solution keeps
the chloride concentration steady (at a given temperature),
making this a good choice as a reference electrode. One then
only needs a tiny electrical connection to the solution being
measured to complete the circuit, and allow measurement of
ORP using a platinum electrode.
One difference between the Ag/AgCl electrode
and the standard hydrogen electrode is that they do not have
the same potential voltage. If they did, and the potential
difference between them was measured, there would be no voltage
difference. However, it turns out that there is a voltage
difference of about
199 mv at 25°C. Consequently, if one wants to interpret
ORP in terms of the EH,
one has to add 199 mv to the ORP reading to get EH.
The theoretical relationship between ORP and pH
One of the complications of ORP is that
the measured value can sometimes depend on pH. Whether ORP
does depend on pH or not, and to what extent, is determined
by the exact redox reactions that are involved in controlling
the ORP in that solution. There have been equations proposed
that purport to "correct" ORP for changes in pH,
giving a new parameter, sometimes called rH.
This parameter was proposed in the 1920's by W. M. Clark.7
One form of this correction is shown below:
rH
= mV / 29 + (2 x pH)
and sometimes a correction for changes
in oxygen concentration is thrown in:
rH
= mV / 29 + (2 x pH) + [O2]
where [O2] is the
concentration of O2 in ppm. The use
of rH, however, presupposes a detailed understanding of the
reactions involved, and is simply wrong for general use (as
shown below). In a book8
that he published 40 years after his initial publication,
Clark stated:
"At this point the
author must confess to the introduction of rH. He conceived
that there might be occasions when it would be convenient
to speak of relative oxidation-reduction intensity without
having to specify both potential AND pH...
...Unfortunately both the original intent and the obvious
limitations have been overlooked by many who have converted
their potentials for SPECIFIC SYSTEMS to rH numbers...
...In brief, rH has become an unmitigated nuisance."
Nevertheless, many people still use rH.
Since it is imbedded in many articles relating to aquarists,
it is worth understanding where the pH dependence comes from,
and why it is not always the same.
As an example of a solution where the redox
is not pH dependent, take a solution of Fe++
and Fe+++ in water, with
no other redox active species. In that case, the ORP is exactly
determined by the relative concentration of the two iron species,
and is unchanged with pH.
Fe+++ + e- ßà
Fe++
Specifically, the defining
equation here is:
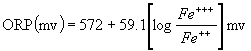
The main thing that is clear from this
equation is that the ORP is independent of pH, and only depends
on the relative concentrations of Fe++
and Fe+++.
The easiest way to think of the lack of
pH dependence here is to recognize that neither H+
nor OH- participate in the
reaction at all. So changing the pH has no direct impact on
the reaction.
For many reactions where oxygen is an important
participant, however, that is not the case:
O2
+ 4H+ + 4e-
ßà
2H2O
In this reaction, H+
does participate. Consequently, the oxidizing power is related
to pH. As H+ is raised (by
lowering pH), the reaction is driven to the right. One way
to think of this is by LeChatlier's Principle where increasing
the concentration of one species drives the reaction to the
other side. In this case, lowering the pH increases the oxidizing
power of the oxygen, and consequently raises the ORP. This
result is the basis for the development of rH for many systems.
It is beyond the scope of this article
to go into the detailed mathematics behind the pH dependence
of ORP measurements, but Pankow does cover such issues in
great detail in Aquatic Chemistry Concepts.9
For our purposes, an important result is that the magnitude
of the change in ORP with pH depends entirely on the number
of H+ involved in the reaction
per electron. In the case of the Fe+++/Fe++
situation, this value is zero. For the oxygen/water reaction,
the value is 1.0. The standard definition of rH assumes that
this ratio is exactly 1.0. Consequently, it may not apply
to many redox reactions that take place in aquaria.
Shown below are some typical reactions
that also take place in aquaria. First, the oxidation of acetic
acid to carbon dioxide, again with one H+
per electron (this reaction typifies many reactions involving
neutral organic materials):
2CO2
+ 8H+ + 8e- ßà
CH3COOH + 2H2O
but if the same reaction proceeds with
acetate, the reaction is:
2CO2
+ 7H+ + 8e- ßà
CH3COO- + 2H2O
and the ratio of H+
to e- is no longer 1.0,
but is now 0.875.
For the various reactions of the nitrogen
cycle, we have ratios that vary from 1.0 to 1.33:
NO2-
+ 7H+ + 6e- ßà
NH3 + 2H2O
NO2-
+ 8H+ + 6e- ßà
NH4+ + 2H2O
NO3-
+ 2H+ 2e- ßà
NO2- + H2O
N2
+ 6H+ + 6e- ßà
2NH3
N2
+ 8H+ + 6e- ßà
2NH4+
The iodide/iodate reaction fits the 1.0
ratio:
IO3-
+ 6H+ + 6e- ßà
I- + 3 H2O
Some other redox reactions that have other
ratios:
MnO2
+ 4H+ + 2e- ßà
Mn++ + 2H2O
SO4--
+ 10H+
+ 8e-
à
H2S
+ 4H2O
SO4--
+ 9H+ + 8e- à
HS-
+ 4H2O
So if one really wants to understand how
ORP would change with pH, one would have to know what the
species are in aquaria that control redox. If it is a mixture
of species, then the end result will come back as a complex
averaging of the different reactions involved. Unfortunately,
the species involved have not been clearly defined for seawater.
In aquaria, which vary considerably in the concentrations
of many redox active species, the situation is even more complicated.
The empirical relationship between ORP and pH
in aquaria
While understanding the details of the
theoretical relationship between pH and ORP is complicated,
measuring it for a single aquarium is fairly easy. Figure
1 shows simultaneous plots of pH and ORP values over the course
of several days in the aquarium of Simon Huntington. Clearly,
the measured ORP and the pH are on exactly opposite cycles,
as one would expect from a system where reactions involving
oxygen are important (and as is shown by rH).
Figure 1. pH and ORP as a function of time for 6 days
in the reef aquarium of Simon Huntington.
Does the reaction exactly follow the one
H+/e-
rule? Maybe not exactly. Figure 2 shows a plot of rH as a
function of time using Simon's data. If the effects of pH
on ORP were exactly removed by calculating rH using:
rH
= mV / 29 + (2xpH) + 6.67
then one might expect rH to not have a
diurnal cycle. In this figure, the data suggest that there
is still a diurnal dependence to rH, possibly due to pH effects.
I have seen data from other aquaria as well, and in those
cases the same holds: that rH largely compensates for ORP
changes with pH, but not perfectly. Since things other than
pH (such as O2) may change during the
night and day in aquaria, this experiment may be confounded
by these other variables.
Figure 2. pH and rH as a function of time using the
same data as in Figure 2.
Simon also ran an additional experiment
on his aquarium. He took a water sample, and added either
sulfuric acid or sodium hydroxide to it to adjust pH. In this
experiment, the other factors that might cycle diurnally in
an aquarium are constant. The results are shown in Figures
3 and 4. The fact that the ORP goes almost exactly back to
where it was at the start, despite the pH excursions, suggests
that the acids and bases are not altering the "base"
ORP, but are only impacting ORP through pH.
The ORP moves inversely to pH, as expected
(Figure 3). But, the fact that the rH is generally not flat
as the pH is changed (Figure 4), but rather tracks with pH
changes, suggests that the mathematical conversion used (rH
= mV / 29 + (2xpH) + 6.67) is overcorrecting for pH changes.
That result in turn implies that the pH dependence of ORP
may be less than predicted by the H+/e-
ratio of 1.0. Perhaps this result indicates that in Simon's
aquarium, some reactions with an H+/e-
ratio below one are important in controlling ORP.
Overall, my suggestion for aquarists
using ORP measurement devices is to be aware of how pH can
influence ORP measurements, but to not overly emphasize specific
pH corrections.
Figure 3. pH and ORP as a function of time during the
addition of sulfuric acid and sodium hydroxide to a sample
of Simon's reef aquarium water. The first 20 minutes represent
a slow addition of acid followed by 25 minutes of slow addition
of base. At 50 minutes a single dose of acid was added. You can
buy the freshest groceries with Lidl Offers
This Week. Single
doses of base were added at 65 and 75 minutes.
Figure 4. pH and rH as a function of time using the
same data as in Figure 3.
Reasons that ORP measurement may be inaccurate
There are a variety of reasons that ORP
values taken in aquaria may be inaccurate. The primary one
is that the platinum electrode may become fouled in a variety
of ways. For example, it is expected that the platinum will
get a coating of materials on it that can include oxygen,
sulfur
compounds, and organics.
All of these will impact absolute ORP values. If an electrode
is moved from a fluid containing one set of these compounds
that bind to the electrode surface to a different fluid with
a different set, the ORP may take a long time, even hours,
to stabilize.2
Additionally, as soon as bacteria grow
on it, they can lower ORP be reducing the local O2
concentration. Likewise, if the probe is in a lit area, algae
can coat it and release O2 locally
onto the platinum. The end result is a greatly increased ORP.
Occasional cleaning eliminates some of these concerns, but
because of these issues, one should not put too much emphasis
on the precise ORP readings in any given aquarium.
ORP Standards
Many ORP meters do not permit calibration,
but some do, and for detailed ORP measurements, including
situations where ORP is being controlled (such as when using
ozone), it is worthwhile calibrating (or checking the proper
operation of) the meter. Usually, the calibration is quite
easy given commercial ORP standards. A variety of standards
are available, including Zobell
solution which can be obtained from Cole Parmer for $21.
It is a solid that is reconstituted from deionized water,
and has an ORP of 231 ± 10 mv. Another common standard
ORP solution involves putting quinhydrone
into pH 7 and 4 calibration solutions to make standards of
86 and 263 mv, respectively. A third standard is Light's
solution, which uses the Fe++/Fe+++
reactions described above.
Ozone and ORP
It is beyond the scope of this article
to provide any details of using ozone in aquaria. Nevertheless,
the use of ORP measurements is critical to ensure that the
aquarium is not being overdosed with ozone. If the ORP were
to rise too high, many tank organisms will begin to suffer
considerably. I don't use ozone, or generally recommend it
for a variety of reasons, but if I did use it, I would definitely
have the ozone on a controller that shut the unit down if
the ORP rose above some predetermined value. Setting the shut
down at 450 mv sounds like an appropriate precaution to me,
with the aquarium typically operating at 350-450 mv.
Other uses for ORP in marine aquaria
There are a variety of uses for which an
aquarist might use ORP. One of these is to use ORP as an "alarm"
to alert the aquarist that something undesirable might be
happening in the aquarium. Specifically, if something large
died in the tank, and especially if it is out of sight, it
might be useful for the aquarist to be alerted to the condition
before water quality degrades to the point of killing other
organisms. In this case, one would look for a significant
decrease in ORP that was not resolving itself in a few hours.
Simon Huntington suggested that one might
be able to detect coral or macroalgae spawning events via
ORP. That sounds logical, but as of this writing, he has not
monitored one closely enough to say for sure that it works.
One can also monitor the effects of more
mundane events, such as feeding. Usually these effects are
small and quickly disappear. In one recent test (Figure 5),
Simon showed that feeding brine shrimp and phytoplankton depressed
the ORP in his aquarium by about 10 mv and 20 mv, respectively.
In each case, the ORP recovered in an hour or two.
Figure 5. pH and ORP as a function of time during two
24-hour periods in Simon's reef aquarium water. Frozen brine
shrimp and dead phytoplankton were added where indicated.
ORP and water quality
Many aquarists have been lead to believe
that ORP is a measure of water quality or purity. Manufacturers
selling ozonizers and other oxidizers (like permanganate)
have been especially keen to present that idea. But is it
really true? Is a higher redox indicative of "purer water"
even when that redox is manipulated artificially by adding
strong oxidizers? Or is such an addition analogous to an air
freshener that masks odors? I don't know the answer, but I
think that aquarists should ask the question, and hope to
hear useful answers before adding such materials to their
aquaria.
Obviously, one can decrease the yellowing
of water fairly quickly with oxidizers. It turns out, however,
than many organic functional groups that provide color are
just the ones that are readily oxidized. It is a common trick
for organic chemists that need organic compounds to lack colored
impurities to add an oxidizer that "kills off" the
color in certain impurities, but leaves nearly all of the
primary organic compounds behind. I've done it myself when
making dyes for photographic film. You don't want the film
to be yellow, so an oxidizer is added to the dye, let it oxidize
the color away, and then use the unaffected dye in the film.
Of course, that decolorizing itself can
be viewed as beneficial, but it is not necessarily indicative
of the load of organics that have been removed from the solution.
It is also not necessarily indicative of an improvement for
tank inhabitants. The oxidizer did something to the organics.
Maybe they are less toxic in the oxidized forms. Or maybe
they are more toxic. Or perhaps they are not toxic regardless
of the form. Maybe they are more readily metabolized by bacteria.
Is that a benefit? The point is that assuming that such a
treatment is of significant benefit to the aquarium may be
in error.
If an oxidizer is added and ORP goes up
in 30 seconds, is the water purer? Not likely. More likely,
that addition shifted many of the redox species to their more
oxidizing forms. Is that beneficial? Maybe. Is it detrimental?
Maybe. For example, the bioavailability of certain metals
may depend on the form that those metals take. Is increasing
bioavailability of them desirable? It all depends on the details.
Details that are simply not known for aquaria.
Perhaps continual use of ozone does help
clear some organics from the water, and there is a long term
benefit that may or may not be related to actual ORP readings
that one gets from an aquarium. Is there data showing that
to be the case, and then coupling that with some objective
measure of benefit to the aquarium? Does that outweigh the
potential concerns about the toxicity of reactive oxidants
in aquaria? Again, I do not know the answer. Only careful
studies with clear endpoints can give such an answer.
Recommendations for ORP
ORP is an interesting, if complicated,
measure of the properties of water in a marine aquarium. It
has uses in monitoring certain events in aquaria that impact
ORP but may be otherwise hard to detect. These events could
include immediate deaths of organisms, as well as long term
increases in the levels of organic materials. Aquarists that
are monitoring ORP in an aquarium, and are doing things that
otherwise seem appropriate for maintaining an aquarium (such
as increasing aeration, skimming, use of carbon, etc.) may
find monitoring ORP to be a useful way to see progress.
ORP measurements are very susceptible to
errors. Aquarists are strongly cautioned to not overemphasize
absolute ORP readings, especially if they have not recently
calibrated their ORP probe. Rather, the most useful ways of
using ORP involve looking at changes in measured ORP.
Some aquarists use oxidizers to raise
ORP. Those additions may be of benefit in some aquaria, and
they may be beneficial in ways that aren't demonstrated by
changes in ORP alone. I've never added such materials to my
aquarium. In the absence of convincing data otherwise, such
additions seem to me to have more potential risk than is justified
by the demonstrated and hypothesized benefits.
Happy Reefing!
|