|
Coral reef decline is a global phenomenon
whose causes are being studied world wide. Especially in the
Caribbean and tropical western Atlantic, this decline is being
greatly structured by increasing frequencies and distribution
of coral diseases (Richardson 1998). Disease impacts not only
the coral species affected, but also the associated reef community.
Human induced stressors, synergistic with disease-causing
organisms, are thought to be the direct or indirect cause
of much coral disease (Bruckner 2002). Environmental stresses,
anthropogenic stresses, microbial pathogens, and other organisms
have all been cited as contributing to or causing coral disease
and mortality (Brown and Howard 1985), yet the etiology of
most coral diseases remains elusive (Richardson 1998). Caribbean
coral reefs have shown a continuing trend towards a phase
shift from coral-dominated to algal-dominated ecosystems with
diseases as one of the primary causes (Lessios et al. 1984,
Aronson and Precht 2001), and such shifts have become a region-wide
concern (Done 1992, Hughes 1994).
The Texas Flower Garden Banks have been
part of a nationally protected marine sanctuary since 1992
and encompass the most northerly and isolated coral reefs
on the North American continental shelf. They are located
approximately 110 nautical miles southeast of Galveston, Texas
in the northwestern Gulf of Mexico. The Flower Garden Banks
have been the subject of research investigations since the
early 1970's and are currently part of extensive monitoring
and management activities. Long-term monitoring has been occurring
since 1972, primarily to assess potential impacts of extensive
oil and gas exploration and industry in the area (Schmahl
and Hickerson 2000). These reefs have been recognized as among
the least disturbed and most pristine coral reef communities
in the Caribbean and Western Atlantic region and possess very
high (44%-55%) coral coverage (Lang 1999, Causey et al. 2000).
Largely unaffected by bleaching events and anthropogenic influences
due to their depth, oceanic inputs, limited recreational users
and isolated location, coral coverage at the Flower Garden
Banks has not significantly declined since monitoring began
in the early 1970's (Lang 1999, Pattengill-Semmens et al.
2000). Coral mortality is primarily due to transient increases
in algal abundance, parrotfish predation, damselfish "lawns,"
and the corallivorous snail Coralliophila abbreviata
(Gittings et al. 1993).
The second most important Caribbean hermatypic
taxon, the Montastraea annularis complex, is currently,
and primarily, threatened by three diseases: black band disease,
yellow blotch disease, and white plague. At the Flower Garden
Banks Montastraea annularis is the dominant species
(29%), followed by Diploria strigosa (9%), Porites
astreoides (5%), and M. cavernosa (4%) (Pattengill-Semmens
et al. 2000). The latter three species are also being impacted
heavily by both black band disease and white plague throughout
the Caribbean and Western Atlantic, and many of the other
20+ hermatypic (reef-building) species present at the Flower
Garden Banks are also susceptible to these pathologies.
The Flower Garden Banks have historically
had a low incidence of coral disease. White mat disease, also
referred to as ridge disease, was first reported there during
monitoring activities from 1988-1991 (Deslarzes 1992). Coral
disease was also monitored as a part of multiple observations
on coral status and health by the Minerals Management Service
(MMS) from 1992-2000 (MMS Publications 96-0046, 99-0005, and
2001-101) across 40 permanent 8m2
quadrats and found to be present at very low levels. "Low"
incidences of disease were reported in 25 coral transects,
with 295 colonies examined during a joint AGRRA/REEF (Reef
Environmental Education Foundation) survey from August 15-20
1999 (Pattengill-Semmens et al. 2000). Other studies put the
incidence of coral disease at less than 2% (Gittings 1995).
In addition to these monitoring projects, there is currently
at least one other researcher doing preliminary investigations
of coral disease at the Flower Gardens Banks (Oberding et
al. 2002).
Unfortunately, many of the reports for
the Flower Garden National Marine Sanctuary (FGNMS) have incorrectly
identified and reported disease levels and conditions. White
mat is not a recognized disease, ridge disease (also called
ridge mortality disease) is probably not a disease but related
to damselfish predation along the ridges of Diploria spp.,
and many researchers have erroneously attributed fish biting,
snail predation, and bleaching events to coral disease (Bruckner
2002). There is also some question that the techniques or
level of expertise developed in previous surveys may not be
sufficient to correctly identify coral disease. In the majority
of cases, gross examination of a specimen at one time point
is inadequate to determine if, or what, disease is present,
and surveys to date have relied on such observations. Samples
for histological examination and determining rates of progression
over time are both important in determining if, and what,
disease(s) might be present. Such sampling has been inadequate
or lacking in previous studies.
During several trips to these sites, I
have documented true coral disease on numerous colonies and
species, and at levels that appear to be higher than in previous
surveys. I have observed unusually high levels numbers of
corals showing signs of hyperplasms erroneously reported as
neoplasms (Oberding et al. 2002)], yellow-blotch disease,
black-band disease, and white plague type II. Furthermore,
several pathologic conditions have been noted that do not
fit the description or known etiology of any currently described
coral disease (Borneman pers. obs.).
Because of the isolation of the Flower
Gardens Banks from land-based influences, gyre currents that
nearly prevent genetic flow from any other reefal areas, low
impact from other stresses, and a healthy baseline condition
of the reefs, incidences of true coral disease at these sites
can provide extremely valuable models of study and are vital
to the preservation of these coral reefs. The large size of
colonies, high coral coverage, and low diversity of hermatypic
species, most of which are known to be susceptible to disease,
make this community especially vulnerable in the event of
a disease epizootic.
The research I will perform has the following
objectives:
a) Conduct rigorous surveys to establish
baseline data documenting the extent of coral diseases throughout
the Flower Garden Banks, including types of disease present,
species affected, percentage of recent mortality, cases
of "false" disease, and descriptions of any new
pathologies.
b) Compare pathologic conditions found
to conditions from other locations showing the same signs
of disease to determine if diseases present at the Flower
Garden Banks are novel or region-wide pathologies.
c) Treat appropriate, large, fecund colonies
affected with coral disease using novel and effective protocols
from other test sites to mitigate the afflicted colonies
and prevent further spread of the disease to other colonies.
d) Begin conducting histological and
molecular investigations to determine cellular processes
of disease, working towards a complete description of the
etiology of the diseases.
The following is the summary and preliminary
report of research conducted on coral disease at the FGNMS
during the spring research cruise from May 26 - May 30, 2003.
I will be doing sufficient research to produce two more preliminary
reports. Subsequent to those, I will write a final summary
report of my findings from this study.
Summary and Impressions
May 27, 2003
Dive 1, Buoy Two, West Bank:
Dive partners: Emma Hickerson, Jenefer Savage
This dive was an overview of the site in
preparation for transects and surveys of coral disease on
the Flower Garden Banks. Locating the Acropora palmata
first observed and photographed in February, 2003, by James
Wiseman and Sarah Bernhardt, was a focus of this dive. It
was found and roughly sited for more careful mapping on a
subsequent dive (Figure 1). No officially named disease was
noticed.
Figure 1. Acropora palmata
A single colony of Montastraea cavernosa
was noted with an opaque white outer "coating" and
somewhat abnormally withdrawn polyps. The appearance was consistent
with other colonies of Faviidae I have seen occasionally in
various places throughout the Caribbean. I have also seen
similar abnormal morphologies in zoanthids. I think there
is a possibility based, in part, on photographs from prior
disease studies, preliminary work on a zoanthid disease by
other workers, and my personal observations as well as analysis
of corals with similar signs, that this may be an external
colonization of the coral surface by the sulfur-oxidizing
bacterial species, Beggiatoa sp. It is my experience
that Beggiatoa colonization can result in polyps becoming
"mushy" and eventually dying in patchy areas across
the corallum. I recommend colonies showing such signs at the
Flower Gardens be monitored to ascertain the pattern of the
condition over space and time, and that samples should be
taken and studied to determine the etiology of this condition.
It is my impression that this pathology does not cause significant
mortality in Scleractinia, nor is it particularly contagious
to adjacent colonies. This may not be true for zoanthids.
Beggiatoa mats are apparently common in deep areas
at the FGNMS. They are nearly ubiquitous in marine environments
and have been identified as single agents or components of
coral diseases; for example, as a consortium member of black
band disease lines and mats. The appearance of colonies affected
by Beggiatoa, given the available reservoirs at the
FGNMS, would not be surprising.
 |
Figure 2. An unidentified white "film" on
Montastraea cavernosa. Compare this
to the normal color pattern shown below.
********
Dive 2, Buoy Two, West Bank:
Dive partners: Emma Hickerson, Jenefer Savage
Impressions: A single 30m transect was
run outward from the U-bolt at mooring 2 towards the identified
colony of Acropora palmata. The colony was located
27m from the U-bolt, in 71 feet of water under an outcropping
topped with a Xestospongia species at a heading of
329º (NNW). A photo transect approximately 2m wide was
made of the line for later analysis, and specific transect
observations noted.
During random swimming of the area surrounding
Mooring 2, we observed an area of Porites astreoides
approximately of 20m2 between
70-80 feet in depth. Most of the colonies in this area displayed
signs of bleaching. This bleaching was expressed as a paling
of tissues. The tissues were not totally transparent and there
was no tissue loss on the colony (Figures 3,4). A quick count
was made of the area and 38 affected colonies were seen. The
bleaching pattern on most colonies appeared to be random,
but most also showed patches or rings of visibly pale tissue
that were more localized towards the colony margins. Generally
affected colonies were over 30% bleached. A sample of a small,
completely affected, colony was photographed and then collected.
The colony was placed in a closed seawater aquarium onboard
the M/V Fling. Porites astreoides located away from
this patch were normally colored, as were other species within
the patch. Bleaching at this depth, temperature and season
is almost certainly not related to thermal stress or irradiance
factors. Current models of bacterial bleaching involving Vibrio
shiloi and V. corallyticus, are positively correlated
with high temperatures. Conditions here would not favor the
likelihood of a similar bleaching pathogen, although the patchy
location, species specificity, and spreading nature of the
condition would tend to indicate that investigation of a possible
pathogen should be considered as an avenue for further study.
Numerous observations of "areas of
abnormal accelerated growth," formerly termed hyperplasm
(sensu Peters), were seen. These areas occurred most commonly
on Diploria strigosa and occasionally on Montastraea
faveolata and M. cavernosa (Figures 5-7). These
"tumors" are common on the Flower Garden Banks and
should be studied further. Their occurrence appears to be
much higher on these reefs than elsewhere in the Caribbean
and Indo-Pacific, and Hickerson's mention of higher than normal
levels of radioactive isotopes occurring at sites in the FGNMS
should be investigated for a possible role in the formation
of these abnormal growth nodules.
Figures
3, 4. An unidentified paling condition in a patchy area
of Porites astreoides.
Figures
5-7. Areas of abnormal accelerated growth, (also referred
to as hyperplasia)
on Diploria strigosa, Copophyllia natans, and
Montastraea cavernosa.
Analysis of the photo transect confirmed
the presence of two small "areas of abnormal accelerated
growth;" one on M. cavernosa and another on D.
strigosa. Also notable on these transects is the apparently
very high rate of fish biting leaving scars. These resulted
in areas of tissue loss ranging from small, local areas to
large widespread areas, and resulting in significant partial
mortality to most species of coral. Also notable is the frequent
and extensive number of colonies that appear to be regrowth
of colonies that had experienced extensive partial, to near
total, old mortality to unknown causes in the past. This transect
contained numerous small colonies indicating relatively high
rates of recruitment. The alga, Lobophora sp., is a
significant contributor to benthic cover where substrate exists
that is not colonized by scleractinians. Frequent "bare
zones" of competition exist between adjacent colonies
and are particularly wide between Diploria and Montastraea
species.
Included on this transect (and throughout
all sites visited on the Flower Garden Banks), were areas
of repeated focus biting by scarids (Figure 8) and areas of
damselfish algal lawn cultivation (Figure 9). The characteristic
appearance of the lesions resulting from specific cases of
fish biting behavior is often distinct, and has falsely led
to the assumption that such lesions were either diseases (at
the Flower Gardens and throughout the Caribbean) or bleaching.
Scarid spot or focused biting was erroneously reported as
Rapid Wasting Syndrome, and damselfish nipping on meandroid
species, such as those in the genus Diploria, have
been called "ridge mortality disease." It is my
impression from prolonged and careful observations here, and
elsewhere, that no primary disease exists that cause these
signs, and that all cases result from current or past action
by the behavioral biting patterns of these reef fish. The
reported higher incidence of the damselfish pattern on Diploria
strigosa at the Flower Gardens deserves further study,
especially insofar as it relates to damsel abundance, predation,
and coral preference; however, the reduced diversity of species
and perhaps their "suitability" to resident damselfish
for algal cultivation may be a factor at these sites. I feel
it is important to note that previous and often flawed surveys
of disease incidence at the FGNMS have included these conditions
in estimating disease prevalence, and managers should be aware
that under current understanding, these lesions should not
be considered as coral diseases.
 |
Figure
8. This parrotfish was observed making the lesion on the
Diploria strigosa
below it, and this has been mistakenly classified as a disease
in some past reports of
disease at the Flower Gardens.
 |
Figure
9. This damselfish (top center of photo) was observed
protecting an algal territory made by nipping the tissue
of the Colpophyllia natans, including meander
ridges. The presence of damselfish was noted at all
of the numerous colonies of Diploria strigosa with
lesions of the ridges of corallites. There were some
colonies without damselfish that had old untended algal
patches and eroded and bare ridges, but these appeared
to have been abandoned by damsels, and the exposed untended
ridges were fouled with other non-turf species indicating
a lack of continued tissue loss. Also note more characteristic
"ridge denuding" at the end of this report.
|
Figure
10. An unidentified white film on M. cavernosa.
A single colony of P. astreoides
exhibiting signs of the "pale condition" described
above appeared on this transect. All other colonies appear
normally pigmented. The corals seen on this transect consisted
of eight species: Mycetophyllia sp., M. faveolata,
M. cavernosa, Madracis mirabilis, M. decactis,
Diploria strigosa, P. astreoides, and Colpophyllia
natans.
********
Dive 3, Buoy Two, West Bank:
Dive partners: Emma Hickerson, Jenefer Savage
A 30 m transect was run approximately due
north from the U-bolt. The results of this transect are listed
below. A small sample of normally pigmented P. astreoides
was sampled as a comparison to the "paled" colony
sampled during Dive #2. Numerous other "paled" P.
astreoides were observed 2-3m from the transect line,
though none occurred within the 2m belt analyzed. Subsequent
to running the transect, a long swim along the "sand
flats" was made, and two colonies of M. cavernosa
and a single colony of M. faveolata were photographed,
all showing signs of the "opaque white film" described
in M. cavernosa mentioned during Dive #1 (Figure 10).
A morph of M. cavernosa displaying a common mottled
white pattern (that is highly fluorescent under actinic or
"black" light) across the colony was also photographed
to compare this "normal" variation with the different
"opaque white" condition (Figure 11). Additionally,
one of the affected colonies also had numerous polyps within
and immediately adjacent to the opaque areas that were visibly
bleached with a yellowish abnormal appearance (Figure 12-13).
This is consistent with similarly affected Montastraea
spp. I have observed at other locations in the Caribbean (Figure
14). During this dive we also observed the common fish biting
patterns and "areas of abnormal accelerated growth"
as described above.
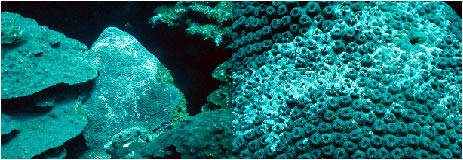 |
Figure
11. Montastraea spp., such as the one shown here
(close-up of same colony shown on right),
and other faviids
often have a highly fluorescent white pattern that is a normal
tissue pattern. The normal
and abnormal patterns appear very
similar at a distance.
Figures
12, 13. The white film seen in Figure 10 seemed to be
related to the corallite paling/yellowing
seen here on the
same and nearby colonies. This condition somewhat resembles
yellow blotch disease,
but it is not the same condition.
Figure
14. A colony of Montastraea at La Parguera, Puerto
Rico, showing similar signs to the
conditions shown in Figures
12, 13.
The corals seen on this transect consisted
of 53 colonies of seven species in seven separate genera:
M. faveolata, D. strigosa, P. astreoides,
Siderastrea siderea, Colpophyllia natans, Millepora
sp., and Stephanocoenia michelini. Two colonies of
D. strigosa showed "areas of abnormal accelerated
growth;" one colony with three nodules ranging from 5cm
to 12 cm in size, the other with a single nodule approximately
4 cm in size.
********
Overall impressions of the West Bank
Disease incidence appears to be very low
with the exception of the unidentified "paling"
condition of P. astreoides and the well-established
incidence of tumor-like areas of abnormal accelerated growth.
Overall, I had an almost intangible feeling that the corals,
in general, on this bank are somehow stressed. Tissue margins
of regrowth following partial mortality were not robust and
the amount of partial mortality resulting from fish biting,
competition, bioerosion and other factors seemed to be increasing
faster than recovery. Coral coverage seemed perceptibly lower
as a result than on previous visits to this bank.
May 28, 2003
Dive 4, Buoy Two, East Bank:
Dive partners: Emma Hickerson, Jenefer Savage
This dive was intended for observation
of large colonies of Stephanocoenia and Madracis
along the deep slope of the East Bank below 120 feet. Colonies
at these deep sites seemed exceptionally healthy and robust.
Shallower areas extending outward from the mooring U-bolt
displayed high coral coverage and with cursory examination
the colonies appeared robust and healthy. Careful observation,
however, revealed a number of conditions that were more varied
than those affecting the West Bank. In particular, several
colonies were observed that appeared to have had black band
disease (M. cavernosa) and white plague (Diploria
strigosa) at some point in the recent past, but were currently
not in a state of active disease progression (Figure 15).
Colonies such as these should be revisited later in the year
as water temperatures increase to see if active disease bands
are formed.
 |
Figure
15. The sharp line of healthy tissue and relatively recently
denuded skeleton on this
Diploria strigosa is indicative
of a disease line. However, some amount of algal overgrowth
had occurred, indicating active tissue loss was not currently
occurring. The large white patchy
area on the front center
of the colony had some corallite erosion, indicative of fish
grazing at
this particular spot. This was the only area of
the skeleton to show septal breakage.
Additionally, an unidentified "pale
ring" condition was observed in both Diploria strigosa
and Colpophyllia natans (Figure 16). This same condition
was observed by Borneman and Bruckner in Puerto Rico in 2001
(Figure 17). The tissue forming a wide ring is visibly paler
than surrounding tissue, although it does not appear to be
severely bleached and no tissue mortality is present. Bruckner
noted that the condition disappeared from Puerto Rico colonies
later that year. This condition does not appear to be a threat
to colony integrity; however, its appearance is abnormal or
unusual and colonies should be observed for increased incidence
or changes in the pathology of affected colonies.
Figure
16. The large, wide pale ring on this Diploria strigosa
was seen on several
colonies, as well as on a single colony
of C. natans. This is an unidentified condition.
Figure
17. A similar unidentified "pale ring" condition
in C. natans, Puerto Rico.
As seen at the West Bank, areas of abnormal
accelerated growth were common on both dives on this bank.
Only a few colonies of P. astreoides were noticed with
any "paling" condition, and their appearance was
questionable enough to suggest that these colonies may not
be affected by the same condition as was seen on the East
Bank; rather, other stressors, shading, fish biting or competition
could all have resulted in the paling. The pale colonies on
this bank were not clustered, either, and the isolation further
suggests that other factors are involved.
********
Dive 5, Buoy Two, East Bank:
Dive partners: Emma Hickerson, Jenefer Savage
This dive was made primarily to collect
samples to establish coral clonal lines, to collect corals
for Dr. Cheryl Woodley's stress marker research, and to collect
an area of abnormal accelerated growth. During the collections,
several additional conditions on the reef were noted. A filamentous,
tuft-like cyanobacteria was present growing on exposed margins
of corals and on reef substrate in an abundance that was considerably
higher than at other sites, and higher than had been previously
observed at the FGNMS. This species occurs commonly in reefs
that are undergoing significant degradation, such as in the
Florida Keys. A gelatinous stranded growth that may be composed
of ciliates (Borneman and Peters) was observed on several
coral colonies (Figure 18). This material is, to my knowledge,
uncharacterized and the role it plays in coral pathology is
unknown. However, its presence, as with the tuft-like cyanobacteria
seems to be correlated with degraded reefs throughout the
Caribbean; its presence at the FGNMS has not been noticed
during my past dives, and its occurrence causes me some degree
of concern and should be investigated further.
 |
Figure
18. A slimy filamentous and unidentified material found
on several coral bommies.
During the sampling of a nodule on a colony
of D. strigosa, a large Xestospongia had bleached
and fallen apart nearby (Figure 19). This condition is a sponge
necrotic disease that is currently affecting sponges throughout
the Caribbean and is a source of significant mortality in
susceptible sponge species, including extremely old and large
sponges. A large colony of D. strigosa was also observed
with a large area of white exposed skeleton with reasonably
defined margins (Figure 20). A cursory glance would indicate
this was an unusually large area of parrotfish focus biting,
but a careful examination showed minimal damage to corallite
septa; this is not usually found after repeated grazing by
scarids. However, the colony lesion was not indicative of
any known disease, either. The fact that this large area of
skeleton was recently denuded of tissue with no algal overgrowth
gave me some degree of consternation. No other corals with
similar lesions were seen, and I was left with a somewhat
questionable impression that it was indeed a result of parrotfish
grazing. Finally, as with other sites, there was a high incidence
of lesions associated with focused biting on various species.
The ridge nipping of meandroid species by damselfish was equally,
if not more, common than at the West Bank.
 |
Figure
19. Xestospongia sp. that had succumbed to a sponge
disease known to
affect similar species throughout the Caribbean.
Figure
20. A suspect area of exposed skeleton on a large Diploria
strigosa colony.
This is probably related to parrotfish
biting, although characteristic grazing scars on
the corallum
were not evident.
Corals collected during this dive included:
two small colonies of healthy P. astreoides, two small
colony fragments of M. faveolata, and a nodule of abnormal
accelerated growth from a colony of D. strigosa (Figure
21-25). These colonies were placed into a closed system aquarium
onboard the M/V Fling.
********
Overall impressions of the East Bank
This area presents somewhat of a paradox
as coral coverage seems higher and coral growth appears to
be more robust than the West Bank. At the same time, there
were isolated incidences of various biotics and diseases that
are correlated with reefs that are under stress and/or are
being degraded in other areas of the greater Caribbean basin.
Because of weather conditions and time constraints, only two
dives were possible here. These dives were also consumed by
project needs that did not allow for transects to be run during
either dive. Future trips to the Flower Garden East bank will
include numerous transects as this bank which needs further
evaluation of disease prevalence. I suspect that disease incidence
will be higher at this site later in the summer as temperatures
continue to increase.
Stetson Bank
Coral coverage and diversity, with the
exception of Millepora sp. and Madracis spp.,
are greatly reduced at Stetson Bank. No disease was found
during any dive, and I have not noted any disease or unusual
undescribed conditions at Stetson Bank in the past.
Overall Trip Statement
The Flower Gardens have historically had
a very low incidence of coral disease, and past reports, surveys,
and analyses may have even overestimated true disease levels.
While historical "baseline" levels of disease in
any ecosystem are expected, these levels are currently unknown
for the most part on coral reefs. The incidence of disease
at the FGNMS is well within what would be expected for species
in other ecosystems, although any level of disease of benthic
long-lived species such as corals might be exceptional. Disease
levels noted in this preliminary report are still considered
"very low," especially in comparison to other areas
of the tropical western Atlantic.
Future trips will incorporate heavier
use of transect data to more accurately quantify levels of
disease present. Nonetheless, coral and sponge disease is
present at the Flower Gardens, and includes: 1)diseases known
from other locations in the Caribbean, 2) abnormal conditions
observed but uncharacterized at other sites in the Caribbean,
and 3) several novel pathologies reported herein. During my
last trip to the FGNMS, I saw several other novel pathologies
that were not seen during this trip. Overall, these reefs
appear to be very healthy, but should be monitored carefully
for any outbreaks or increased reports of anomalous conditions
affecting the benthic species. The nature of this reserve,
both in habitat, location, environmental parameters, and isolation
would suggest that any epizootic disease outbreak could have
implications that would negatively impact reef diversity and
structure perhaps to an even greater extent than most other
more common and typical reef systems in the region. In particular,
the novel characteristics of the reef system at the FGNMS
provide an important opportunity to study and understand the
numerous pathologies affecting these reefs and others throughout
the Caribbean.
I feel it is also important to ensure that
anyone involved in assessing coral disease at the FGNMS in
the future be familiar with this reef system and with coral
pathologies to avoid confounding reports that misidentify
conditions and lesions, or misrepresent what is and is not
"normal." Clearly, FGNMS managers must play an integral
role because of the remoteness of this area and the limited
familiarity that most persons have with the sanctuary. Managers
may be among the only people familiar enough with the FGNMS
to be able to accurately assess changes occurring over the
short term. I think that our future trips associated with
the work described in this current report will prove enlightening
and will provide a much more complete picture of coral disease
at the FGNMS, especially if, and when, they are coupled with
more complete analysis of collected samples. Once an accurate
assessment of disease conditions and incidence is available,
managers should be in a much better position to monitor and
report changes to what is, unfortunately, a still very incomplete
picture of coral disease at the Flower Gardens.
Stetson Bank Coral List Notes
Siderastrea sp.
-Both S. radians and S. siderea
exist at Stetson and I saw numerous colonies of both.
Stephanocoenia intersepta
-I'm not sure this is valid. They are all
synonymized as S. michelini by both Veron and Humann.
Agaricia fragilis?
-I will get species designations for all the agariciids on the next trip here and at the Flower Gardens
Figures
The following photos were taken during
the current research cruise to the Flower Gardens and may
represent anomalous conditions. The signs seen in the photos
are unstudied and uncharacterized, to my knowledge.
An unusual color pattern in M. cavernosa.
Although I have seen colonies with similar patterns
throughout
the Caribbean, and no colony mortality has been noted. I am
unsure if this is a normal
or pathological condition or appearance
for corals showing such patterns or signs.
A similar anomalous color pattern in a
Montastraea faveolata colony.
An anomalous color pattern with slight
paling in a deepwater Sidearastrea siderea. The patterns
here may be indicative of fish biting. However, odd color
patterns like this are seen in numerous
genera and are seemingly
more common at the Flower Gardens than other locations in
the tropical
western Atlantic. For example, see photos below.
Left:
parrotfish focused biting, erroneously called rapid wasting
disease on M. faveolata.
Right: damselfish lawns, erroneously called ridge mortality
disease on D. strigosa.
Left:
possible white plague Type II on on D. strigosa.
Center: Colpophyllia natans with white plague,
not at the Flower Gardens, for comparison.
Right: An area of abnomal accelerated growth on M.
cavernosa.
Left:
unknown pathology affecting C. natans.
Right: unknown pathology affecting M. faveolata.
Cyanobacterial tufts are found sporadically
at the West Bank.
Another unusual color pattern common at
the Flower Gardens.
|